Theoretical Physics T2 Quantum Mechanics
... of light on particles, called Compton effect or Compton scattering, see Fig. 1.8. For a description we study energy and momentum of the photon, both particle properties. The effect works in total analogy to a scattering process with particles, i.e. energy and momentum are conserved. ...
... of light on particles, called Compton effect or Compton scattering, see Fig. 1.8. For a description we study energy and momentum of the photon, both particle properties. The effect works in total analogy to a scattering process with particles, i.e. energy and momentum are conserved. ...
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
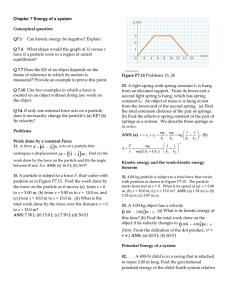
... potential energy of its system depends on its position r as graphed in Figure P8.46. In the limit as r increases without bound, U(r) approaches +1 J. (a) Identify each equilibrium position for this particle. Indicate whether each is a point of stable, unstable or neutral equilibrium. (b) The particl ...
... potential energy of its system depends on its position r as graphed in Figure P8.46. In the limit as r increases without bound, U(r) approaches +1 J. (a) Identify each equilibrium position for this particle. Indicate whether each is a point of stable, unstable or neutral equilibrium. (b) The particl ...
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
... particle within a system due to its intteaction with the rest of the system. The equation Fx = (2x + 4) N describes the force, where x is in meters. As the particle moves along the x axis from x = 1.00 m to x = 5.00 m, calculate (a) the work done by this force, (b) the change in the potential energy ...
... particle within a system due to its intteaction with the rest of the system. The equation Fx = (2x + 4) N describes the force, where x is in meters. As the particle moves along the x axis from x = 1.00 m to x = 5.00 m, calculate (a) the work done by this force, (b) the change in the potential energy ...
Problem 1. Kinematics of the Lambda decays
... The lambda particle (Λ) is a neutral baryon of mass M = 1115 MeV that decays with a lifetime of τ = 2.9 × 10−10 s into a nucleon of mass m1 = 939 MeV and a π-meson of mass m2 = 140 MeV. It was first observed by its charged decay mode Λ → p + π − in cloud chambers. In the clould chamber (and in detec ...
... The lambda particle (Λ) is a neutral baryon of mass M = 1115 MeV that decays with a lifetime of τ = 2.9 × 10−10 s into a nucleon of mass m1 = 939 MeV and a π-meson of mass m2 = 140 MeV. It was first observed by its charged decay mode Λ → p + π − in cloud chambers. In the clould chamber (and in detec ...
lect22
... variables” which we do not know but which gives the system an underlying deterministic structure. We hide our ignorance by describing the “most probable” outcomes of measurement ...
... variables” which we do not know but which gives the system an underlying deterministic structure. We hide our ignorance by describing the “most probable” outcomes of measurement ...
Light - UDChemistry
... • It is impossible to know both the position and momentum of an electron simultaneously • The only way to know anything about an electron is to shoot it with a photon • The photon alters the position and/or momentum in an unpredictable manner, so the original position and ...
... • It is impossible to know both the position and momentum of an electron simultaneously • The only way to know anything about an electron is to shoot it with a photon • The photon alters the position and/or momentum in an unpredictable manner, so the original position and ...
Quantum Harmonic Oscillator
... "quantized", and may only take the discrete values of times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not ...
... "quantized", and may only take the discrete values of times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not ...
PHB - Indian Statistical Institute
... 3. Differential equations : ODE − Existence of solution, fundamental system of integrals, elementary notions, special functions. PDE upto second order, equations of parabolic, hyperbolic and elliptic type. 4. Dynamics of particles and rigid bodies : Motion of a particle in a plane and on a smooth cu ...
... 3. Differential equations : ODE − Existence of solution, fundamental system of integrals, elementary notions, special functions. PDE upto second order, equations of parabolic, hyperbolic and elliptic type. 4. Dynamics of particles and rigid bodies : Motion of a particle in a plane and on a smooth cu ...
Physical Science EOCT Review Domain IV Waves, Electricity and
... • EM spectrum shows the forms of radiation in order of increasing frequency (and energy) and ...
... • EM spectrum shows the forms of radiation in order of increasing frequency (and energy) and ...
Physics 2018: Great Ideas in Science: The Physics Module Quantum
... 3. Since Newtonian and Maxwellian physics describe the macroscopic world so well, physicists developing quantum mechanics demanded that when applied to macroscopic systems, the new physics must reduce to the old physics =⇒ this Correspondence Principle was coined by Niels Bohr. 4. Due to quantum me ...
... 3. Since Newtonian and Maxwellian physics describe the macroscopic world so well, physicists developing quantum mechanics demanded that when applied to macroscopic systems, the new physics must reduce to the old physics =⇒ this Correspondence Principle was coined by Niels Bohr. 4. Due to quantum me ...
Powerpoint handout
... Niels Bohr explained all the various lines by proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...
... Niels Bohr explained all the various lines by proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...