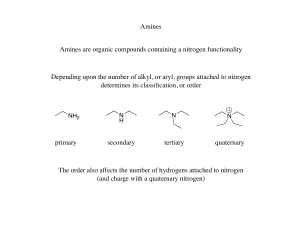
Chapter 20
... Identifying Redox Reactions In general, all chemical reaction can be assigned to one of two classes 1. Redox reactions in which electrons are transferred from one reacting species to another. a. Many single-replacement reactions, combination reactions, decomposition reactions and combustion reactio ...
... Identifying Redox Reactions In general, all chemical reaction can be assigned to one of two classes 1. Redox reactions in which electrons are transferred from one reacting species to another. a. Many single-replacement reactions, combination reactions, decomposition reactions and combustion reactio ...
Chapter 19
... The amide does not generally react further because the lone pair of electrons is delocalized ...
... The amide does not generally react further because the lone pair of electrons is delocalized ...
Chlorotrimethylsilane/Sodium Iodide, a
... periods of reaction times, although in such cases propene can be used as a n acid scavenger. Experimental Section Starting Materials. All the starting materials used in this work were either commercially available in generally 98%or higher purity and used without further purification or prepared by ...
... periods of reaction times, although in such cases propene can be used as a n acid scavenger. Experimental Section Starting Materials. All the starting materials used in this work were either commercially available in generally 98%or higher purity and used without further purification or prepared by ...
enantioselective zeolite-catalyzed reactions
... major topic of discussion and research.1 Homogeneous, asymmetry-inducing catalysts have been developed for a wide range of reactions, especially notable are those developed by Knowles, Noyori and Sharpless, for which they jointly received the 2001 Nobel Prize in Chemistry.2 Although these reactions ...
... major topic of discussion and research.1 Homogeneous, asymmetry-inducing catalysts have been developed for a wide range of reactions, especially notable are those developed by Knowles, Noyori and Sharpless, for which they jointly received the 2001 Nobel Prize in Chemistry.2 Although these reactions ...
Q4) How the following conversions can be carried out?
... b. Here—OH group is predominant and the alcohol molecules can form hydrogen bonds with water molecules. Q8. Give reasons: i)Nitration of phenol gives ortho- and para- products only. ii)Why do alcohols have higher boiling points than the haloalkanes of the same molecular mass? ANS (1) -OH group incre ...
... b. Here—OH group is predominant and the alcohol molecules can form hydrogen bonds with water molecules. Q8. Give reasons: i)Nitration of phenol gives ortho- and para- products only. ii)Why do alcohols have higher boiling points than the haloalkanes of the same molecular mass? ANS (1) -OH group incre ...
Structures of Escherichia coli Branched
... substrate-PLP configurations which are mirror images of each other. The orientation of a bound substrate in eBCAT is the same as that in AspAT, and that of PLP is the same as that in bsDAAT. The important residues and hydrogen bonds for the catalytic action are conserved in these enzymes. The cataly ...
... substrate-PLP configurations which are mirror images of each other. The orientation of a bound substrate in eBCAT is the same as that in AspAT, and that of PLP is the same as that in bsDAAT. The important residues and hydrogen bonds for the catalytic action are conserved in these enzymes. The cataly ...
Kool Colors
... reaction. The steel wool in this activity loses electrons and is said to be oxidized. Students can observe this by looking at the steel wool afterward. The steel wool may turn black or may show signs of rust. Steel is primarily made from iron, and, when iron oxidizes, we call it rust. If something l ...
... reaction. The steel wool in this activity loses electrons and is said to be oxidized. Students can observe this by looking at the steel wool afterward. The steel wool may turn black or may show signs of rust. Steel is primarily made from iron, and, when iron oxidizes, we call it rust. If something l ...
Chapter 21 aldehydes and ketones
... • Since phosphorus is a second-row element, it can be surrounded by more than eight electrons. • Thus, a second resonance structure can be drawn that places a double bond between carbon and phosphorus. • Regardless of which resonance structure is drawn, a Wittig reagent has no net charge. • However, ...
... • Since phosphorus is a second-row element, it can be surrounded by more than eight electrons. • Thus, a second resonance structure can be drawn that places a double bond between carbon and phosphorus. • Regardless of which resonance structure is drawn, a Wittig reagent has no net charge. • However, ...
JSUNIL TUTORIAL , SAMASTIPUR, BIHAR
... IUPAC (International Union of Pure and Applied Chemistry) approves names of elements. Many of the symbols are the first one or two letters of the element‟s name in English. The first letter of a symbol is always written as a capital letter (uppercase) and the second letter as a small letter (lowerca ...
... IUPAC (International Union of Pure and Applied Chemistry) approves names of elements. Many of the symbols are the first one or two letters of the element‟s name in English. The first letter of a symbol is always written as a capital letter (uppercase) and the second letter as a small letter (lowerca ...
What is a mole? - Chemical Paradigms
... ‘One mole of methane reacts with two moles of oxygen gas to produce [or yield] one mole of carbon dioxide gas and two moles of water. Heat energy is released.’ The arrow represents produces or yield. Scientists never put an equal (=) sign instead of the arrow. The substances reacting are called reac ...
... ‘One mole of methane reacts with two moles of oxygen gas to produce [or yield] one mole of carbon dioxide gas and two moles of water. Heat energy is released.’ The arrow represents produces or yield. Scientists never put an equal (=) sign instead of the arrow. The substances reacting are called reac ...
Lab announcements – 2 lab quiz week before spring break
... photocopy before turning in or study before turning in -same as 101/other 102 sections be extra careful with dilutions this week (Expt. 21) Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur simultaneously at the same rate ‘equilibrium’ doesn’t neces ...
... photocopy before turning in or study before turning in -same as 101/other 102 sections be extra careful with dilutions this week (Expt. 21) Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur simultaneously at the same rate ‘equilibrium’ doesn’t neces ...
18-19 SpontEnt
... Nature tends to move spontaneously from a state of lower probability to one of higher probability »!G.N. Lewis (Nobel Laureate) ...
... Nature tends to move spontaneously from a state of lower probability to one of higher probability »!G.N. Lewis (Nobel Laureate) ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
Advantages of racemic DNA crystallography
... as a major limiting factor for structure determination. Small organic molecules prefer to crystallize in space group in which it is easiest to fill space. Larger molecules such as biomolecules, crystallizes primarily in space groups in which it is easiest to achieve connectivity. The strong tendency ...
... as a major limiting factor for structure determination. Small organic molecules prefer to crystallize in space group in which it is easiest to fill space. Larger molecules such as biomolecules, crystallizes primarily in space groups in which it is easiest to achieve connectivity. The strong tendency ...
Document
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
University Grants Commission New Delhi Proposed Syllabus and Scheme of Examination
... Polymer waste management ...
... Polymer waste management ...
Chapter 3 Stoichiometry: Calculations with Chemical
... 12. How many moles of carbon atoms are in 4 mol of dimethylsulfoxide (C2H6SO)? (a). 2 (b). 6 (c). 8 (d). 4 Explanation: This is based on reading the formula and correctly extracting information from it. The formula C2H6SO indicates that every mole of this compound has 2 moles of carbon atoms in it. ...
... 12. How many moles of carbon atoms are in 4 mol of dimethylsulfoxide (C2H6SO)? (a). 2 (b). 6 (c). 8 (d). 4 Explanation: This is based on reading the formula and correctly extracting information from it. The formula C2H6SO indicates that every mole of this compound has 2 moles of carbon atoms in it. ...