12. Structure Determination: Mass Spectrometry and
... The MS of propane is more complex (Figure 12-2 b) since the molecule can break down in several ways ...
... The MS of propane is more complex (Figure 12-2 b) since the molecule can break down in several ways ...
12. Structure Determination: Mass Spectrometry and Infrared
... The MS of propane is more complex (Figure 12-2 b) since the molecule can break down in several ways ...
... The MS of propane is more complex (Figure 12-2 b) since the molecule can break down in several ways ...
Chapter 9 Notes - Get a Clue with Mrs. Perdue
... Could lead to organ failure, blindness, death, etc. ...
... Could lead to organ failure, blindness, death, etc. ...
2.10 Organic synthesis – Oxidation of alcohols
... • The reaction will proceed at a constant temperature (i.e. the solvent's boiling point.). •Any vapours given off are cooled back to liquid, and fall back into the reaction vessel • Useful for performing chemical reactions under controlled conditions that require substantial time for completion. ...
... • The reaction will proceed at a constant temperature (i.e. the solvent's boiling point.). •Any vapours given off are cooled back to liquid, and fall back into the reaction vessel • Useful for performing chemical reactions under controlled conditions that require substantial time for completion. ...
Transition Metal Chemistry 2 2011.12.2 Ⅰ Fundamental
... 4-1 CO Insertion mechanism in Mn-Me bond: alkyl migration Principle of microscopic reversibility in chemical reaction: Forward and reverse reactions must have the same transition state.----Analysis of reverse reaction (de-insertion) gives information of transition state of insertion reaction. (Simil ...
... 4-1 CO Insertion mechanism in Mn-Me bond: alkyl migration Principle of microscopic reversibility in chemical reaction: Forward and reverse reactions must have the same transition state.----Analysis of reverse reaction (de-insertion) gives information of transition state of insertion reaction. (Simil ...
Chapter 4 Carbon Chemistry
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
c - Tan Lam
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
elements of chemistry unit
... 3. The arrangement of atoms in molecules and polyatomic ions is depicted by: (A) abbreviated configurations (B) configurations (C) condensed structural diagrams (D) orbital diagrams (E) structural diagrams 4. Two atoms share four electrons. What type of bond exists between the two atoms? (A) single ...
... 3. The arrangement of atoms in molecules and polyatomic ions is depicted by: (A) abbreviated configurations (B) configurations (C) condensed structural diagrams (D) orbital diagrams (E) structural diagrams 4. Two atoms share four electrons. What type of bond exists between the two atoms? (A) single ...
1 - vnhsteachers
... 3. The arrangement of atoms in molecules and polyatomic ions is depicted by: (A) abbreviated configurations (B) configurations (C) condensed structural diagrams (D) orbital diagrams (E) structural diagrams 4. Two atoms share four electrons. What type of bond exists between the two atoms? (A) single ...
... 3. The arrangement of atoms in molecules and polyatomic ions is depicted by: (A) abbreviated configurations (B) configurations (C) condensed structural diagrams (D) orbital diagrams (E) structural diagrams 4. Two atoms share four electrons. What type of bond exists between the two atoms? (A) single ...
10. Alkyl Halides
... X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses ...
... X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses ...
M. Sc. Semester -I CHE401 Inorganic Chemistry
... 1. Calibration of glass wares and balance. 2. Calibration of pH meter, conductometer and potentiometer. 3. Determination of nicotine in tobacco (non-aqueous titration). 4. Determination of available chlorine in bleaching powder. 5. Determination of vitamin C in orange juice/amla. 6. Determination of ...
... 1. Calibration of glass wares and balance. 2. Calibration of pH meter, conductometer and potentiometer. 3. Determination of nicotine in tobacco (non-aqueous titration). 4. Determination of available chlorine in bleaching powder. 5. Determination of vitamin C in orange juice/amla. 6. Determination of ...
1 - M*W
... gas. Which of the following represents the reactants in this reaction? a) Magnesium and magnesium chloride b) Hydrochloric acid and hydrogen gas c) Magnesium and hydrochloric acid d) Magnesium chloride and hydrogen gas 50) The equation E=mc2 shows that a) Chemical reactions are either exothermic or ...
... gas. Which of the following represents the reactants in this reaction? a) Magnesium and magnesium chloride b) Hydrochloric acid and hydrogen gas c) Magnesium and hydrochloric acid d) Magnesium chloride and hydrogen gas 50) The equation E=mc2 shows that a) Chemical reactions are either exothermic or ...
Snc2d Chapter 5 Practice Test
... b) In the diagram above, the Roman group number of P shows: c) The period number of P shows: d) Show a Bohr diagram above of P forming an ion, indicating beside your diagram the number of electrons gained or lost. Include the symbol with net charge and the name of the ion formed. e) With regard to i ...
... b) In the diagram above, the Roman group number of P shows: c) The period number of P shows: d) Show a Bohr diagram above of P forming an ion, indicating beside your diagram the number of electrons gained or lost. Include the symbol with net charge and the name of the ion formed. e) With regard to i ...
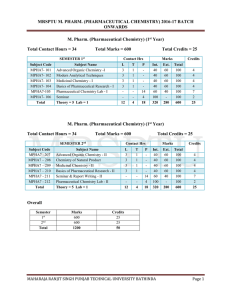
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... 5. J. Mendham, R.C. Denney, J.D. Barnes and M. Thomas, ‘Vogel’s Textbook of Quantitative Chemical Analysis’, Pearson Education Limited, Singapore. 6. R.M. Silverstein and F.X. Webster, ‘Spectrometric Identification of Organic Compounds’, John Wiley and Sons, New York. 7. Eliel and H. Samuel, ‘Stereo ...
... 5. J. Mendham, R.C. Denney, J.D. Barnes and M. Thomas, ‘Vogel’s Textbook of Quantitative Chemical Analysis’, Pearson Education Limited, Singapore. 6. R.M. Silverstein and F.X. Webster, ‘Spectrometric Identification of Organic Compounds’, John Wiley and Sons, New York. 7. Eliel and H. Samuel, ‘Stereo ...
Parallel Computing in Chemistry
... what program to use? how to build/optimize that program for a platform how to effectively run the program on the machine identification of common pitfalls (and pratfalls, too) ...
... what program to use? how to build/optimize that program for a platform how to effectively run the program on the machine identification of common pitfalls (and pratfalls, too) ...
Chemistry_in_Parallel_Computing_old
... what program to use? how to build/optimize that program for a platform how to effectively run the program on the machine identification of common pitfalls (and pratfalls, too) ...
... what program to use? how to build/optimize that program for a platform how to effectively run the program on the machine identification of common pitfalls (and pratfalls, too) ...
Marvelous Macromolecules
... ____________ bonds Hydrophobic/hydrophilic interactions Van der Waals interactions ___________ bonds (charged R groups) Disulfide bridges between _____________ groups of cysteine amino acids (stabilize structure) ...
... ____________ bonds Hydrophobic/hydrophilic interactions Van der Waals interactions ___________ bonds (charged R groups) Disulfide bridges between _____________ groups of cysteine amino acids (stabilize structure) ...
Topic2890 Thermodynamics and Kinetics A given system at
... which chemists understand in terms of spontaneous chemical reaction (in a closed system). The task for chemists is to identify the actual chemical reaction in a given closed system. Thermodynamics and Chemical Kinetics ...
... which chemists understand in terms of spontaneous chemical reaction (in a closed system). The task for chemists is to identify the actual chemical reaction in a given closed system. Thermodynamics and Chemical Kinetics ...
04- carbon chemistry text
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
... Carbon Chemistry • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds ...
Document
... A limitless variety of polymers can be built from a small set of monomers • Inherent differences between siblings result from variations in polymers • Construction of macromolecules – 40-50 common monomers and others that occur rarely – Small molecules that are common to all organisms are ordered i ...
... A limitless variety of polymers can be built from a small set of monomers • Inherent differences between siblings result from variations in polymers • Construction of macromolecules – 40-50 common monomers and others that occur rarely – Small molecules that are common to all organisms are ordered i ...
Review Questions
... 5. Will a precipitate form in each of the following cases? Use Ksp tables to justify your answer. a. 50 mL of 0.040 mol/L Ca(NO3)2 (aq) plus 150 mL of 0.080 mol/L (NH4)2SO4 (aq) b. 10 mL of 0.005 mol/L AgNO3 (aq) plus 25 mL of 0.02 mol/L NaBr (aq) 6. If the [H+(aq)] in a solution is 3.0 x 10-4 mol/L ...
... 5. Will a precipitate form in each of the following cases? Use Ksp tables to justify your answer. a. 50 mL of 0.040 mol/L Ca(NO3)2 (aq) plus 150 mL of 0.080 mol/L (NH4)2SO4 (aq) b. 10 mL of 0.005 mol/L AgNO3 (aq) plus 25 mL of 0.02 mol/L NaBr (aq) 6. If the [H+(aq)] in a solution is 3.0 x 10-4 mol/L ...