Organic Chemistry
... THOMAS POON is Professor of Chemistry in the W.M. Keck Science Department of Claremont McKenna, Pitzer, and Scripps Colleges, three of the five undergraduate institutions that make up the Claremont Colleges in Claremont, California. He received his B.S. degree from Fairfield University (CT) and his ...
... THOMAS POON is Professor of Chemistry in the W.M. Keck Science Department of Claremont McKenna, Pitzer, and Scripps Colleges, three of the five undergraduate institutions that make up the Claremont Colleges in Claremont, California. He received his B.S. degree from Fairfield University (CT) and his ...
Slide 1
... - The quantity of product predicted by stoichiometry the theoretical yield - the amount actually obtained the actual yield Percent yield = (actual yield) / (theoretical yield) (100%) ...
... - The quantity of product predicted by stoichiometry the theoretical yield - the amount actually obtained the actual yield Percent yield = (actual yield) / (theoretical yield) (100%) ...
Chemistry Comes Alive: Part A
... • Unequal sharing by atoms with different electron-attracting abilities produces polar molecules • H2O • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium ...
... • Unequal sharing by atoms with different electron-attracting abilities produces polar molecules • H2O • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium ...
chemistry - cloudfront.net
... How many grams of mercury would you have if you had 32 cm3 of mercury? The density of mercury is 13.6 g/cm3. D = m/V m = D x V = 13.6 g/cm3 x 32 cm3 = 440 g Ronnie has a piece of aluminum which has a mass of 10.8 grams and a volume of 4 cubic centimeters. Calculate the density of aluminum. D = ...
... How many grams of mercury would you have if you had 32 cm3 of mercury? The density of mercury is 13.6 g/cm3. D = m/V m = D x V = 13.6 g/cm3 x 32 cm3 = 440 g Ronnie has a piece of aluminum which has a mass of 10.8 grams and a volume of 4 cubic centimeters. Calculate the density of aluminum. D = ...
Molecular Models Activity
... provide information concerning the actual arrangement of atoms in the molecule with their correct geometry. In other words, not every molecule is as flat as we draw then on paper when we determine a structural formula. Structural formulas only give some information about the real arrangement of atom ...
... provide information concerning the actual arrangement of atoms in the molecule with their correct geometry. In other words, not every molecule is as flat as we draw then on paper when we determine a structural formula. Structural formulas only give some information about the real arrangement of atom ...
C - Milwaukie High
... Organic Reactions combustion of hydrocarbons OR compounds w/only C, H, and O: products are…CO2 and H2O Write the equation for the complete combustion of 2-methyl-2-pentene. C6H12 + 9 O2 6 CO2 + 6 H2O ...
... Organic Reactions combustion of hydrocarbons OR compounds w/only C, H, and O: products are…CO2 and H2O Write the equation for the complete combustion of 2-methyl-2-pentene. C6H12 + 9 O2 6 CO2 + 6 H2O ...
What is BIOCHEMISTRY?
... McKee & McKee: "the study of the molecular basis of life" Therefore, biochemistry must focus upon the molecules and chemical reactions that occur within cells. ...
... McKee & McKee: "the study of the molecular basis of life" Therefore, biochemistry must focus upon the molecules and chemical reactions that occur within cells. ...
Worksheet 9b - Department of Chemistry | Oregon State University
... The decomposition of 18F follows first order kinetics (i.e. Rate = k[18F]) and has a halflife of 1.83 days. The initial amount of a fluorine-18 is 20.0 g. How much time must pass for 1.00 g to remain? ...
... The decomposition of 18F follows first order kinetics (i.e. Rate = k[18F]) and has a halflife of 1.83 days. The initial amount of a fluorine-18 is 20.0 g. How much time must pass for 1.00 g to remain? ...
Syracuse University
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
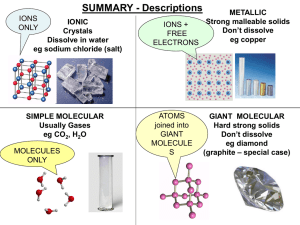
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
AP® Chemistry
... B. Structural, geometric, optical, and conformational isomerism of 1. Organic molecules 2. Coordination complexes VI. Polarity of molecules VII. Relation of molecular structure to physical properties The student will: 1. Draw Lewis structures for the common atoms, ions, and molecules. 2. Use periodi ...
... B. Structural, geometric, optical, and conformational isomerism of 1. Organic molecules 2. Coordination complexes VI. Polarity of molecules VII. Relation of molecular structure to physical properties The student will: 1. Draw Lewis structures for the common atoms, ions, and molecules. 2. Use periodi ...
Physical chemistry exam, quiz, homework with Solution
... 19. The ground state of the ozone molecule O3 has the following shape (A) linear (B) bent (C) equilateral triangle (D) tetrahedral (E) trigonal bipyramid (B) 20. Sulfur apparently shows a valence of 6 in the molecule SF6 , whereas oxygen, just above it in the periodic table, has only a valence of 2. ...
... 19. The ground state of the ozone molecule O3 has the following shape (A) linear (B) bent (C) equilateral triangle (D) tetrahedral (E) trigonal bipyramid (B) 20. Sulfur apparently shows a valence of 6 in the molecule SF6 , whereas oxygen, just above it in the periodic table, has only a valence of 2. ...
Atomic Structure (27 Jan 2004) • What is matter? • Dalton`s Atomic
... - transfer of electrons from metals and nonmetals - ions held together by electrostatic forces • Ionic solids and their properties • Metallic Bonding and properties of metals • Packing in ionic and metallic solids • Covalent Bonding - sharing of electrons between nonmetals ...
... - transfer of electrons from metals and nonmetals - ions held together by electrostatic forces • Ionic solids and their properties • Metallic Bonding and properties of metals • Packing in ionic and metallic solids • Covalent Bonding - sharing of electrons between nonmetals ...
CHM_101_ASSIGNMENT_COPY_1_2
... attraction of the positive nucleus for the electron will increase. More energy is needed to remove the outermost electron, thus the ionization energy increases. 2. Size of the positive nuclear charge: As the nuclear charge increases, its attraction for the outermost electron increases, and so more ...
... attraction of the positive nucleus for the electron will increase. More energy is needed to remove the outermost electron, thus the ionization energy increases. 2. Size of the positive nuclear charge: As the nuclear charge increases, its attraction for the outermost electron increases, and so more ...
Organic Agriculture`s Research Team
... Ravneet joined the research team in January 2015 as a graduate student majoring in Plant Science. She is conducting leafy green variety trials in the open field and in the hoop house to determine optimum choices for the southern United States’ farmers and producers. Parameters include total yield, w ...
... Ravneet joined the research team in January 2015 as a graduate student majoring in Plant Science. She is conducting leafy green variety trials in the open field and in the hoop house to determine optimum choices for the southern United States’ farmers and producers. Parameters include total yield, w ...
Hydrocarbon Worksheet - Building Aliphatic
... HYDROCARBONS Carbon atoms have the unique ability to forms long chains while retaining their ability to bond covalently with other elements. Aside from carbon and hydrogen, organic compounds may also contain oxygen, nitrogen, sulfur, chlorine, bromine, and iodine. According to its Lewis dot diagram, ...
... HYDROCARBONS Carbon atoms have the unique ability to forms long chains while retaining their ability to bond covalently with other elements. Aside from carbon and hydrogen, organic compounds may also contain oxygen, nitrogen, sulfur, chlorine, bromine, and iodine. According to its Lewis dot diagram, ...