013368718X_CH28_437-452.indd
... cell processes, form cellular structures, carry substances into or out of cells, and help fight ...
... cell processes, form cellular structures, carry substances into or out of cells, and help fight ...
CHM 103 Lecture 11 S07
... Reaction Rate and Catalysts A catalyst • increases the rate of a reaction. • lowers the energy of activation. • is not used up during the reaction. ...
... Reaction Rate and Catalysts A catalyst • increases the rate of a reaction. • lowers the energy of activation. • is not used up during the reaction. ...
Chem Bonding Notes
... (1) both solids contain only ionic bonds (2) both solids contain only covalent bonds (3) solid A contains only covalent bonds and solid B contains only ionic bonds (4) solid^4 contains only ionic bonds and solid B contains only covalent bonds 33. In the space provided in your answer booklet, draw an ...
... (1) both solids contain only ionic bonds (2) both solids contain only covalent bonds (3) solid A contains only covalent bonds and solid B contains only ionic bonds (4) solid^4 contains only ionic bonds and solid B contains only covalent bonds 33. In the space provided in your answer booklet, draw an ...
2nd nine weeks benchmark review homework
... On the periodic table, an element is arranged by it ___which is equal to the number of ___ in the nucleus. a- atomic number, neutrons b- atomic number, protons c- atomic mass, neutrons d- atomic mass, protons When the physical composition of a substance changes, the chemical composition- ...
... On the periodic table, an element is arranged by it ___which is equal to the number of ___ in the nucleus. a- atomic number, neutrons b- atomic number, protons c- atomic mass, neutrons d- atomic mass, protons When the physical composition of a substance changes, the chemical composition- ...
Functional Groups
... Functional Groups A specific arrangement of atoms in an organic compound that is capable of characteristic chemical reactions. In other words, a substituent group other than an alkyl group. Most organic chemistry is functionalgroup chemistry. We will do one functional group. ...
... Functional Groups A specific arrangement of atoms in an organic compound that is capable of characteristic chemical reactions. In other words, a substituent group other than an alkyl group. Most organic chemistry is functionalgroup chemistry. We will do one functional group. ...
Class Syllabus Ch120a: Nature of the Chemical Bond Units: 3-0
... Courses in QM often focus more on applied mathematics rather than physical concepts. We start by understanding some of the essential differences between quantum and classical mechanics, one of which is the description of kinetic energy. These ideas are used to understand why atoms are stable and wh ...
... Courses in QM often focus more on applied mathematics rather than physical concepts. We start by understanding some of the essential differences between quantum and classical mechanics, one of which is the description of kinetic energy. These ideas are used to understand why atoms are stable and wh ...
Chapter 1 Chemistry: The Study of Matter
... States of matter Solid- matter that has definite shape (can not flow) and has definite volume. Liquid- definite volume but takes the shape of its container (flows). Gas- a substance without definite volume or shape (can flow). Vapor- a substance that is currently a gas but normally is a liquid ...
... States of matter Solid- matter that has definite shape (can not flow) and has definite volume. Liquid- definite volume but takes the shape of its container (flows). Gas- a substance without definite volume or shape (can flow). Vapor- a substance that is currently a gas but normally is a liquid ...
Notes
... meaning that the ends are connected to form a regular geometric shape. Ex. triangle, square, pentagon, hexagon, etc. Benzene is not a cyclic alkane due to its double bonds and resonance. Prefix: Cyclo Examples: Cyclopropane, C3H6 Cyclobutane, C4H8 ...
... meaning that the ends are connected to form a regular geometric shape. Ex. triangle, square, pentagon, hexagon, etc. Benzene is not a cyclic alkane due to its double bonds and resonance. Prefix: Cyclo Examples: Cyclopropane, C3H6 Cyclobutane, C4H8 ...
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
... Propanol which is an alcohol and thus contains the OH group will also display hydrogen bonding. Thinking back the higher course each of the intermolecular attractions will give rise to differences in both the physical and chemical properties of a molecule. Boiling Points—most organic molecules conta ...
Chapter 4: Carbon and the Molecular Diversity of Life
... Carbon atoms are the most versatile building blocks of molecules (pp. 53-55, FIGURE 4.2) A covalent-bonding capacity of four contributes to carbon’s ability to form diverse molecules. Carbon can bond to a variety of atoms, including O, H, and N. Carbon atoms can also bond to other carbons, forming ...
... Carbon atoms are the most versatile building blocks of molecules (pp. 53-55, FIGURE 4.2) A covalent-bonding capacity of four contributes to carbon’s ability to form diverse molecules. Carbon can bond to a variety of atoms, including O, H, and N. Carbon atoms can also bond to other carbons, forming ...
BIOLOGY Chapter 3 Test – Chemistry of Life
... 21. Which of the following statements is NOT a true characteristic of water? a. Water is cohesive b. Water absorbs a great deal of heat before it evaporates. c. Water is adhesive d. Liquid water is less dense than ice. 22. Water has a neutral pH of a. 0 b. 2 c 7 d. 14 23. The pH of your blood hovers ...
... 21. Which of the following statements is NOT a true characteristic of water? a. Water is cohesive b. Water absorbs a great deal of heat before it evaporates. c. Water is adhesive d. Liquid water is less dense than ice. 22. Water has a neutral pH of a. 0 b. 2 c 7 d. 14 23. The pH of your blood hovers ...
AP Chem Stoichiometry Topic#4 Questions WS Name: Date: Per
... If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical formula of the original compound? (b) Could you draw a diagram representing the molecules of the compound that had been decomposed? Why or why not? (6) The diagram represents the collection of CO2 and H2 ...
... If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical formula of the original compound? (b) Could you draw a diagram representing the molecules of the compound that had been decomposed? Why or why not? (6) The diagram represents the collection of CO2 and H2 ...
212Final`97
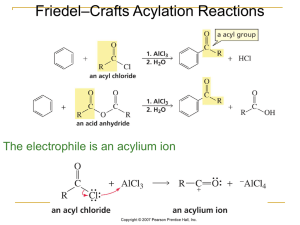
... a) CH3Cl with AlCl3; then KMnO4; then HNO3 / H2SO4 b) CH3Cl with AlCl3; then HNO3 / H2SO4; then KMnO4 c) HNO3 / H2SO4; then KMnO4; then CH3Cl with AlCl3 d) HNO3 / H2SO4; then CH3Cl with AlCl3; then KMnO4 17. (4) Which of the following sequences gives the compound shown at the left in the highest yie ...
... a) CH3Cl with AlCl3; then KMnO4; then HNO3 / H2SO4 b) CH3Cl with AlCl3; then HNO3 / H2SO4; then KMnO4 c) HNO3 / H2SO4; then KMnO4; then CH3Cl with AlCl3 d) HNO3 / H2SO4; then CH3Cl with AlCl3; then KMnO4 17. (4) Which of the following sequences gives the compound shown at the left in the highest yie ...
3_2: More Chemical Changes
... Investigate: Chemical Reactions • In today’s lab, you will be looking at chemical reactions that occur between 8 different solid materials. The solids have been dissolved in water to make solutions. ...
... Investigate: Chemical Reactions • In today’s lab, you will be looking at chemical reactions that occur between 8 different solid materials. The solids have been dissolved in water to make solutions. ...
Chapter 4
... • Vitamins are organic compounds needed in small amounts for normal cell function. • Most cannot be synthesized in our bodies, and must be obtained from the diet. • Most are identified by a letter, such as A, C, D, E, and K. • There are several different B vitamins, so a subscript is added to distin ...
... • Vitamins are organic compounds needed in small amounts for normal cell function. • Most cannot be synthesized in our bodies, and must be obtained from the diet. • Most are identified by a letter, such as A, C, D, E, and K. • There are several different B vitamins, so a subscript is added to distin ...
CHAPTER-6 DEHYDROHALOGENATION OF ALKYL HALIDES
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
CLASSIFICATION OF MATTER AND CHEMICAL AND PHYSICAL
... c. Wood(s) + O2(g) → CO2(g) + H2O(l) chemical 2. Iodine is a solid with somewhat lustrous, blue-black crystals. The crystals vaporize readily to a violetcolored gas. Iodine, like chlorine, combines with many metals. For example, aluminum combines with iodine to give aluminum iodide. Identify each o ...
... c. Wood(s) + O2(g) → CO2(g) + H2O(l) chemical 2. Iodine is a solid with somewhat lustrous, blue-black crystals. The crystals vaporize readily to a violetcolored gas. Iodine, like chlorine, combines with many metals. For example, aluminum combines with iodine to give aluminum iodide. Identify each o ...
DALTON`S ATOMIC THEORY - 1808: Publication of Dalton`s "A New
... Not according to Dalton's theory. Dalton's theory would predict a different RATIO of water and oxygen would form: ...
... Not according to Dalton's theory. Dalton's theory would predict a different RATIO of water and oxygen would form: ...
File
... of alcohols, carbonyls, esters and carboxylic acids. Describe how shielding influences the chemical shift of an atom. Explain why spin-spin coupling is seen in 1H nmr but not in 13C nmr. State the typical range of the δ scale for 13C nmr. ...
... of alcohols, carbonyls, esters and carboxylic acids. Describe how shielding influences the chemical shift of an atom. Explain why spin-spin coupling is seen in 1H nmr but not in 13C nmr. State the typical range of the δ scale for 13C nmr. ...