Surfactants
... Surfactants are a class of molecules with ‘dual’ or ‘two-faced’ physical natures. • One end is polar • The other end is non-polar So these amphipathic molecules are able to interact with (or be soluble in) both polar and non-polar molecules & solvents. :O: Soaps are a good example: ...
... Surfactants are a class of molecules with ‘dual’ or ‘two-faced’ physical natures. • One end is polar • The other end is non-polar So these amphipathic molecules are able to interact with (or be soluble in) both polar and non-polar molecules & solvents. :O: Soaps are a good example: ...
CH 3
... • Identify the largest hydrocarbon group attached to the N atom as the parent alkane. • Replace the –e at the end with the new ending – amine. Include a position number, if necessary, to show the location of the functional group on the hydrocarbon chain • Name the other alkyl group(s) attached to th ...
... • Identify the largest hydrocarbon group attached to the N atom as the parent alkane. • Replace the –e at the end with the new ending – amine. Include a position number, if necessary, to show the location of the functional group on the hydrocarbon chain • Name the other alkyl group(s) attached to th ...
File
... 54. In which species is the electron geometry around the central atom tetrahedral? A) SF4 B) BF4– C) XeF4 D) PCl5 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HC ...
... 54. In which species is the electron geometry around the central atom tetrahedral? A) SF4 B) BF4– C) XeF4 D) PCl5 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HC ...
1 - 嘉義大學
... (A) It would double its value. (B) It would become half its current value. (C) It would quadruple its value. (D) It would not change its value. 21. What statement about equilibrium is true? (A) When two opposing processes proceed at identical rates, the system is at equilibrium. (B) The equilibrium ...
... (A) It would double its value. (B) It would become half its current value. (C) It would quadruple its value. (D) It would not change its value. 21. What statement about equilibrium is true? (A) When two opposing processes proceed at identical rates, the system is at equilibrium. (B) The equilibrium ...
CHEM 203 Important Topics for Review – See Chapters 1
... Important Topics for Review – See Chapters 1-3 of Brown-Foote-Iverson Chapter 1 Covalent bonding in organic molecules Covalent bonding as "electron-sharing" between atomic pairs Particularly stable electronic configuration of inert (= noble) gases Principle: atoms in a bonded state tend to acquire a ...
... Important Topics for Review – See Chapters 1-3 of Brown-Foote-Iverson Chapter 1 Covalent bonding in organic molecules Covalent bonding as "electron-sharing" between atomic pairs Particularly stable electronic configuration of inert (= noble) gases Principle: atoms in a bonded state tend to acquire a ...
Organic compounds
... Draw Figure 3.5 phospholipid. Label the head and the tail. 1. How would the polar head of a phospholipid respond to water molecules? 2. How would the nonpolar tails respond to water molecules? ...
... Draw Figure 3.5 phospholipid. Label the head and the tail. 1. How would the polar head of a phospholipid respond to water molecules? 2. How would the nonpolar tails respond to water molecules? ...
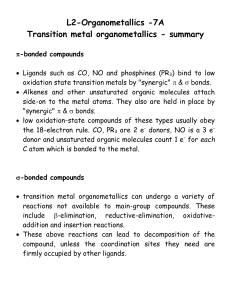
lect7
... The metal atom is "holding" the reactive species together. Step 3. Insertion of alkene into Co-H bond Hydride H atom adds to C atom of alkene H(RCH=CH2)Co(CO)3 + CO RCH2CH2 - Co(CO)4 (18 e) Step 4. Insertion of CO into Co-alkyl bond RCH2CH2Co(CO)4 + CO RCH2CH2 - C(O) - Co(CO)4 (18 e) ...
... The metal atom is "holding" the reactive species together. Step 3. Insertion of alkene into Co-H bond Hydride H atom adds to C atom of alkene H(RCH=CH2)Co(CO)3 + CO RCH2CH2 - Co(CO)4 (18 e) Step 4. Insertion of CO into Co-alkyl bond RCH2CH2Co(CO)4 + CO RCH2CH2 - C(O) - Co(CO)4 (18 e) ...
Exam 3 Review - Iowa State University
... a. What are the formal charges of Cl, N, and O in each structure? b. Are any of the oxygen atoms equivalent? c. What nitrogen- and oxygen-containing ion is isoelectronic with ClNO2? 22. Which contains a multiple bond in its Lewis structure? a. ICl b. SO2 c. Cl2 d. NH4+ e. CaI2 23. Find the enthalpy ...
... a. What are the formal charges of Cl, N, and O in each structure? b. Are any of the oxygen atoms equivalent? c. What nitrogen- and oxygen-containing ion is isoelectronic with ClNO2? 22. Which contains a multiple bond in its Lewis structure? a. ICl b. SO2 c. Cl2 d. NH4+ e. CaI2 23. Find the enthalpy ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... In addition to natural photosynthesis, CO2 can be also utilized by artificial chemical processes designed by chemical engineers. In these processes, CO2 is converted to various useful organic and inorganic substances such as fuels, fertilizers, polymer and construction materials. 4. Write one reacti ...
... In addition to natural photosynthesis, CO2 can be also utilized by artificial chemical processes designed by chemical engineers. In these processes, CO2 is converted to various useful organic and inorganic substances such as fuels, fertilizers, polymer and construction materials. 4. Write one reacti ...
72KB
... bonded to three other C atoms in a 2-D or layered arrangement with weak intermolecular forces of attraction between the layers or sheets. In diamond, the covalent bonds between the carbon atoms are very strong and hold the atoms in place, making it difficult to break the bonds. Therefore diamond is ...
... bonded to three other C atoms in a 2-D or layered arrangement with weak intermolecular forces of attraction between the layers or sheets. In diamond, the covalent bonds between the carbon atoms are very strong and hold the atoms in place, making it difficult to break the bonds. Therefore diamond is ...
Chemistry
... describe, interpret and/or predict the effect of different types of bonding (ionic bonding; covalent bonding; hydrogen bonding; other intermolecular interactions; metallic bonding) on the physical properties of substances ...
... describe, interpret and/or predict the effect of different types of bonding (ionic bonding; covalent bonding; hydrogen bonding; other intermolecular interactions; metallic bonding) on the physical properties of substances ...
File
... 6 = hex 3 = prop 7 = hept 4 = but 8 = oct Suffix is determined by the type of bond Alkane CnH2n+2 (all bonds are single) Alkene CnH2n (one bond is a double) Alkyne CnH2n-2 (one bond is a triple) ...
... 6 = hex 3 = prop 7 = hept 4 = but 8 = oct Suffix is determined by the type of bond Alkane CnH2n+2 (all bonds are single) Alkene CnH2n (one bond is a double) Alkyne CnH2n-2 (one bond is a triple) ...
Organic Molecules: Introduction and key concepts
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
... In organic chemistry, a functional group is a speci c group of atoms within molecules, that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a par ...
Chemistry Standard Course of Study -- Detailed - UNCG GK-12
... COMPETENCY GOAL 2: The learner will build an understanding of the structure and properties of matter. (34%) ...
... COMPETENCY GOAL 2: The learner will build an understanding of the structure and properties of matter. (34%) ...
CHEMICAL REACTION
... • Sometimes it is easier to balance water as HOH instead of H2O (if OH1- ion is involved). ...
... • Sometimes it is easier to balance water as HOH instead of H2O (if OH1- ion is involved). ...
Polymers
... 2. Write names and structural formulas for basic alkanes, alkenes, alkynes and cyclic compounds. 3. Identify isomers of given organic compounds. 4. Identify, write names and structures for compounds containing common types of functional groups. 5. Differentiate between polymers and monomers. 6. Diff ...
... 2. Write names and structural formulas for basic alkanes, alkenes, alkynes and cyclic compounds. 3. Identify isomers of given organic compounds. 4. Identify, write names and structures for compounds containing common types of functional groups. 5. Differentiate between polymers and monomers. 6. Diff ...
Unit C
... formulas, using International Union of Pure and Applied Chemistry (IUPAC) nomenclature guidelines, for saturated and unsaturated aliphatic (including cyclic) and aromatic carbon compounds • containing up to 10 carbon atoms in the parent chain (e.g., pentane; 3ethyl-2,4- dimethylpentane) or cyclic st ...
... formulas, using International Union of Pure and Applied Chemistry (IUPAC) nomenclature guidelines, for saturated and unsaturated aliphatic (including cyclic) and aromatic carbon compounds • containing up to 10 carbon atoms in the parent chain (e.g., pentane; 3ethyl-2,4- dimethylpentane) or cyclic st ...