*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
AP Chemistry Syllabus – Joliet Township High School
... apply the seven science practices defined in the AP Chemistry Curriculum Framework. At minimum, six of the required 16 labs are conducted in a guided-inquiry format. The course provides opportunities for students to develop, record, and maintain evidence of their verbal, written, and graphic communi ...
... apply the seven science practices defined in the AP Chemistry Curriculum Framework. At minimum, six of the required 16 labs are conducted in a guided-inquiry format. The course provides opportunities for students to develop, record, and maintain evidence of their verbal, written, and graphic communi ...
Part (d) The Birch Reduction of Nitrogen
... Clearly there is a problem in making ketones with this chemistry. Three solutions are available. 1) React a carboxylic acid with TWO equivalents of a reactive organolithium reagent ...
... Clearly there is a problem in making ketones with this chemistry. Three solutions are available. 1) React a carboxylic acid with TWO equivalents of a reactive organolithium reagent ...
class 2.pptx
... Chlorine is a mixture of two isotopes : 35Cl, 75.8%, and 37Cl, 24.2%. Chlorine occurs as Cl2 molecules. A mass spectrometer can be used to measure the mass of molecules - not bulk samples. In this case, this is done by making Cl2+ ions and using their charge-to-mass ratios to distinguish the masses ...
... Chlorine is a mixture of two isotopes : 35Cl, 75.8%, and 37Cl, 24.2%. Chlorine occurs as Cl2 molecules. A mass spectrometer can be used to measure the mass of molecules - not bulk samples. In this case, this is done by making Cl2+ ions and using their charge-to-mass ratios to distinguish the masses ...
b) How many electrons are in carbons 2nd energy
... 1 of 20) Three part question: a) What is the atomic number of Carbon? b) How many combined protons and neutrons are inside the nucleus? c) How many electrons are in carbon’s 2nd electron level? ...
... 1 of 20) Three part question: a) What is the atomic number of Carbon? b) How many combined protons and neutrons are inside the nucleus? c) How many electrons are in carbon’s 2nd electron level? ...
class 2.pptx
... neutrons, but the same number of protons in their nuclei and the same number of electrons have essentially identical ...
... neutrons, but the same number of protons in their nuclei and the same number of electrons have essentially identical ...
8. Chemistry of cooking
... butanone (a) Name the two products formed by the dehydration of butan-2-ol (b) Name a reagent which could be used to oxidise butan-2-ol to butanone. ...
... butanone (a) Name the two products formed by the dehydration of butan-2-ol (b) Name a reagent which could be used to oxidise butan-2-ol to butanone. ...
File
... The metals iron, zinc, copper, silver, and lead commonly occur in their compounds as Fe2+, Fe3+, Zn2+, Cu2+, Ag+, and Pb2+ ions. (2) ...
... The metals iron, zinc, copper, silver, and lead commonly occur in their compounds as Fe2+, Fe3+, Zn2+, Cu2+, Ag+, and Pb2+ ions. (2) ...
Chemistry Midterm Review Sheet
... Listed below is a detailed outline of each of these areas to help you study. However, even if something is not specifically listed below, it is still fair game. Your notes, old problem sets, and tests will prove invaluable in helping to study for the exam. In terms of the textbook, we have covered C ...
... Listed below is a detailed outline of each of these areas to help you study. However, even if something is not specifically listed below, it is still fair game. Your notes, old problem sets, and tests will prove invaluable in helping to study for the exam. In terms of the textbook, we have covered C ...
Study guide - cloudfront.net
... Ethanol and dimethyl ether, structural isomers, have the same number and kinds of atoms but a different boding sequence and very different properties. Maleic acid and fumaric acid are geometric isomers whose double bonds fix the spatial arrangement of the molecule. Maleic acid is the cis isomer; bot ...
... Ethanol and dimethyl ether, structural isomers, have the same number and kinds of atoms but a different boding sequence and very different properties. Maleic acid and fumaric acid are geometric isomers whose double bonds fix the spatial arrangement of the molecule. Maleic acid is the cis isomer; bot ...
Jeopardy Macros
... 1. Name all the indicators used in the labs we have done, and list the negative control and positive controls for each and what specific macromolecule they test for ...
... 1. Name all the indicators used in the labs we have done, and list the negative control and positive controls for each and what specific macromolecule they test for ...
World of Carbon Flashcards
... atoms and one hydrogen atom, producing a planar, hexagonal ring. This leaves a ’spare’ electron on each carbon atom. These electrons are able to move from atom to atom around the ring. ...
... atoms and one hydrogen atom, producing a planar, hexagonal ring. This leaves a ’spare’ electron on each carbon atom. These electrons are able to move from atom to atom around the ring. ...
Physical Science Chapter 6
... beginning substances (on the left) called reactants; ending substances (on the right) called products; arrow in the middle means “yields” or “gives”. ...
... beginning substances (on the left) called reactants; ending substances (on the right) called products; arrow in the middle means “yields” or “gives”. ...
File
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
APBiologySummerisOVERAssignment
... 3. List 5 other elements naturally found in the body. 4. Distinguish between protons, neutrons, and electrons. 5. Write out the electron configuration for an atom of fluorine in the ground state. 6. How are radioactive isotopes useful in biology? 7. What is the difference between kinetic and potenti ...
... 3. List 5 other elements naturally found in the body. 4. Distinguish between protons, neutrons, and electrons. 5. Write out the electron configuration for an atom of fluorine in the ground state. 6. How are radioactive isotopes useful in biology? 7. What is the difference between kinetic and potenti ...
File
... d. none of these 2. The solvent in an aqueous solution is always a. Oxygen b. an acid c. a base d. water 3. A solid produced during a chemical reaction is called a (an) a. solute b. precipitate c. spectator ion d. solvent 4. Balance the following equation. Fe(s) + H2O(l) Fe2O3(s) + H2(g) 5. Balanc ...
... d. none of these 2. The solvent in an aqueous solution is always a. Oxygen b. an acid c. a base d. water 3. A solid produced during a chemical reaction is called a (an) a. solute b. precipitate c. spectator ion d. solvent 4. Balance the following equation. Fe(s) + H2O(l) Fe2O3(s) + H2(g) 5. Balanc ...
Chemical Reactions
... formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. ( ...
... formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. ( ...
www.tutor-homework.com (for tutoring, homework help, or help with
... The maximum number of electrons that can be accommodated in a p subshell is a. 2. b. 4. c. 6. d. 10. e. 8. ...
... The maximum number of electrons that can be accommodated in a p subshell is a. 2. b. 4. c. 6. d. 10. e. 8. ...
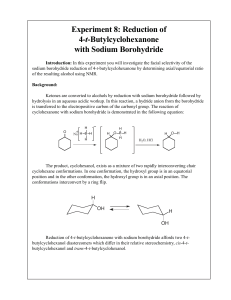
Experiment 8: Reduction of 4-t-Butylcyclohexanone with Sodium
... liquids separate into two layers or “phases” with the more dense liquid on the bottom. As a general rule of thumb, if the organic solvent is halogenated (dichloromethane) it will be more dense than water and thus on the bottom. If it is non-halogenated (ether, hexane, ethyl acetate) it will be less ...
... liquids separate into two layers or “phases” with the more dense liquid on the bottom. As a general rule of thumb, if the organic solvent is halogenated (dichloromethane) it will be more dense than water and thus on the bottom. If it is non-halogenated (ether, hexane, ethyl acetate) it will be less ...
2 Other Organic Compounds
... Other Organic Compounds • Table R – for most of these you will use Table R for examples ...
... Other Organic Compounds • Table R – for most of these you will use Table R for examples ...
Chemical Reactions - TSHSChemistry
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...