Organic Chemistry II Introduction
... Widely used as automobile antifreeze (lowers freezing point of water solutions) ...
... Widely used as automobile antifreeze (lowers freezing point of water solutions) ...
Exam 3 - Napa Valley College
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
The d-Block Elements
... the overall trends are rather common, however, and in many cases, they are attributable to the stability associated with filled and half-filled subshells. For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configur ...
... the overall trends are rather common, however, and in many cases, they are attributable to the stability associated with filled and half-filled subshells. For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configur ...
PC_Chemistry_Macomb_April08
... The integrity of the scientific process depends on scientists and citizens understanding and respecting the “Nature of Science.” Openness to new ideas, skepticism, and honesty are attributes required for good scientific practice. Scientists must use logical reasoning during investigation design, ana ...
... The integrity of the scientific process depends on scientists and citizens understanding and respecting the “Nature of Science.” Openness to new ideas, skepticism, and honesty are attributes required for good scientific practice. Scientists must use logical reasoning during investigation design, ana ...
AP Chemistry - Notes
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
No Slide Title
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
physicochemical properties of organic medicinal agents
... Perhaps the most noteworthy difference between alkenes/alkenyl groups and polar organic functionality in terms of drug chemistry is the difference in solubility properties. As discussed in other tutorials, structurally similar or analogous compounds (“like” compounds) display overlapping solubility ...
... Perhaps the most noteworthy difference between alkenes/alkenyl groups and polar organic functionality in terms of drug chemistry is the difference in solubility properties. As discussed in other tutorials, structurally similar or analogous compounds (“like” compounds) display overlapping solubility ...
+ H 2 O(l) - Knockhardy
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
CHAPTER 19
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
GRE Chemistry Test Practice Book
... other modern main group and transition metal reagents and catalysts F. Special Topics — Resonance, molecular orbital theory, catalysis, acid-base theory, carbon acidity, aromaticity, antiaromaticity, macromolecules, lipids, amino acids, peptides, carbohydrates, nucleic acids, terpenes, asymmetric s ...
... other modern main group and transition metal reagents and catalysts F. Special Topics — Resonance, molecular orbital theory, catalysis, acid-base theory, carbon acidity, aromaticity, antiaromaticity, macromolecules, lipids, amino acids, peptides, carbohydrates, nucleic acids, terpenes, asymmetric s ...
Alcohols Phenols and Ethers
... bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydroxyl group occurs in a carboxylic acid, aldehyde, or ketone, it is named as a substit ...
... bond and one for the hydroxyl group. The –ol suffix is last and takes precedence in the numbering. 3. If the hydroxyl group is directly attached to an aromatic ring, the compound is named as a phenol. 4. If the hydroxyl group occurs in a carboxylic acid, aldehyde, or ketone, it is named as a substit ...
Review Study Guide for the Final
... What is it called when you have more electrons than protons? ...
... What is it called when you have more electrons than protons? ...
14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
... When making assumptions, if a reaction has a relatively small keq and a relatively large initial reactant concentration, then the concentration change (x) can often be neglected without introducing significant error. This does not mean x = 0, because then this would mean there is no reaction. It mea ...
01. Introduction of bioorganic chemistry. Classification, structure
... Kekule’s formula: carbon atoms in a benzene molecule are arranged in a six-membered ring with one hydrogen atom bonded to each carbon atom and with three double carbon-carbon bonds. Benzene doesn’t react as a typical alkene (doesn’t decolorize bromine solution, negative Bayer’s test). Benzene behave ...
... Kekule’s formula: carbon atoms in a benzene molecule are arranged in a six-membered ring with one hydrogen atom bonded to each carbon atom and with three double carbon-carbon bonds. Benzene doesn’t react as a typical alkene (doesn’t decolorize bromine solution, negative Bayer’s test). Benzene behave ...
Benzylamine reacts with nitrous acid to form unstable
... 1. Write the use of quaternary ammonium salts? 2. What product is formed when aniline is first diazotized and then treated with Phenol in alkaline medium? 3. How is phenyl hydrazine prepared from aniline? 4. What is the IUPAC name of a tertiary amine containing one methyl, one ethyl and one n-propyl ...
... 1. Write the use of quaternary ammonium salts? 2. What product is formed when aniline is first diazotized and then treated with Phenol in alkaline medium? 3. How is phenyl hydrazine prepared from aniline? 4. What is the IUPAC name of a tertiary amine containing one methyl, one ethyl and one n-propyl ...
Unit 5: Oragnic Chemistry Notes (answers)
... 1. Most Alkenes and Alkynes are Non-Polar. They only have London Dispersion Forces. 2. Their boiling points are comparable to alkanes. The double bonds and triple bonds do NOT have any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compar ...
... 1. Most Alkenes and Alkynes are Non-Polar. They only have London Dispersion Forces. 2. Their boiling points are comparable to alkanes. The double bonds and triple bonds do NOT have any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compar ...
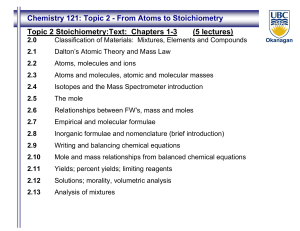
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined solely from its atomic number. For example, the atomic number of nitrogen is ...
... ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of protons is equal to the number of electrons ¾ The chemical identity of an atom can be determined solely from its atomic number. For example, the atomic number of nitrogen is ...
Predissociation dynamics of lithium iodide
... reactions with laser pulses has initiated the field of coherent control.1–3 The idea is to selectively control the outcome of photo-induced reactions—formation of a chemical bond or creation of photofragments—by changing the parameters of the laser pulses. Femtosecond pump-probe spectroscopy has bee ...
... reactions with laser pulses has initiated the field of coherent control.1–3 The idea is to selectively control the outcome of photo-induced reactions—formation of a chemical bond or creation of photofragments—by changing the parameters of the laser pulses. Femtosecond pump-probe spectroscopy has bee ...
Unit X Organic Chem (SmartBoard)
... The benzene ring, also known as an “AROMATIC RING”, is present in a large number of molecules and many molecules contain two or more aromatic rings joined together. An AROMATIC MOLECULE is a molecule containing one or more benzene rings. NAMING AROMATIC MOLECULES: i) ...
... The benzene ring, also known as an “AROMATIC RING”, is present in a large number of molecules and many molecules contain two or more aromatic rings joined together. An AROMATIC MOLECULE is a molecule containing one or more benzene rings. NAMING AROMATIC MOLECULES: i) ...
Developments in Synthetic Application of Selenium(IV) Oxide and
... 2. Selenium(IV) Oxide and Selenic(IV) Acid as Oxidizing Agents and Oxidation Catalysts The first publication on the use of selenium(IV) oxide in oxidation reactions appeared in 1932 [27] and since then it has been applied as a versatile reagent for the synthesis of various types of organic compounds ...
... 2. Selenium(IV) Oxide and Selenic(IV) Acid as Oxidizing Agents and Oxidation Catalysts The first publication on the use of selenium(IV) oxide in oxidation reactions appeared in 1932 [27] and since then it has been applied as a versatile reagent for the synthesis of various types of organic compounds ...