ap chemistry 2005/2006
... connected to find the voltages generated. The values are used to contract a table of “relative” electrode potentials. Also, the change in concentration of one of the solutions will be observed to see how this affects cell potential. Thirdly, we will determine the solubility product of silver chlorid ...
... connected to find the voltages generated. The values are used to contract a table of “relative” electrode potentials. Also, the change in concentration of one of the solutions will be observed to see how this affects cell potential. Thirdly, we will determine the solubility product of silver chlorid ...
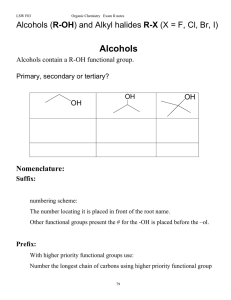
Reaction of Alkenes
... The reaction is regioselective The reaction is regioselective (alcohol on the least‐substituted carbon) (alcohol on the least substituted carbon) and stereoselective (syn‐addition) ...
... The reaction is regioselective The reaction is regioselective (alcohol on the least‐substituted carbon) (alcohol on the least substituted carbon) and stereoselective (syn‐addition) ...
1 H NT Ch 12—Stoichiometry I. Review: Chemical Equations a
... ii. Predict and balance the reaction of solid aluminum with hydrochloric acid. What mass of hydrogen can be produced if you start with 6.000 mol of aluminum? ...
... ii. Predict and balance the reaction of solid aluminum with hydrochloric acid. What mass of hydrogen can be produced if you start with 6.000 mol of aluminum? ...
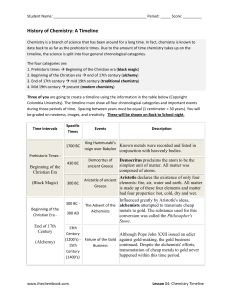
Role of Chemistry in Everyday Life
... series of chemical reactions using enzymes, to change complex chemicals in food to end products that can be absorbed by the body's cells. The caffeine in the coffee and tea we drink affects our mood and keeps us awake. These are all chemical through a chemical change, which is why cooked food often ...
... series of chemical reactions using enzymes, to change complex chemicals in food to end products that can be absorbed by the body's cells. The caffeine in the coffee and tea we drink affects our mood and keeps us awake. These are all chemical through a chemical change, which is why cooked food often ...
Notes matter energy
... indicate the number of atoms in the formula (when only one atom of a given type is present, no ‘1’ is written). The chemical formula can be written from a description of the composition. For example, the molecule niacin consists of 6 carbon atoms, 6 hydrogen atoms, 2 nitrogen atoms, and 1 oxygen ato ...
... indicate the number of atoms in the formula (when only one atom of a given type is present, no ‘1’ is written). The chemical formula can be written from a description of the composition. For example, the molecule niacin consists of 6 carbon atoms, 6 hydrogen atoms, 2 nitrogen atoms, and 1 oxygen ato ...
Exam I - Chemistry With BT
... subsequently goes missing. They do remember the H(combustion) values to be different for their samples. In order to help the researchers, please construct the energy diagram below filling the boxes with the appropriate reactants and/or products. ...
... subsequently goes missing. They do remember the H(combustion) values to be different for their samples. In order to help the researchers, please construct the energy diagram below filling the boxes with the appropriate reactants and/or products. ...
Measurements/Unit Cancellation/Significant Figures 1. When
... Empirical formula: A chemical formula that shows the lowest relative number of atoms of each element in a compound. Formula mass: The sum of the atomic masses (atomic weights in amu) of the atomic species as given in the formula of the compound Hydrate: A compound in which a specific number of water ...
... Empirical formula: A chemical formula that shows the lowest relative number of atoms of each element in a compound. Formula mass: The sum of the atomic masses (atomic weights in amu) of the atomic species as given in the formula of the compound Hydrate: A compound in which a specific number of water ...
9. E1: Alkenes from alcohols - Web Pages
... Background Elimination reactions. In this experiment, cyclohexanol will be dehydrated (loss of H2O) to form cyclohexene under acidic conditions (see Figure 1). OH ...
... Background Elimination reactions. In this experiment, cyclohexanol will be dehydrated (loss of H2O) to form cyclohexene under acidic conditions (see Figure 1). OH ...
File - Grade 12 Chemistry
... 9. ANS: Dispersion forces are very weak intermolecular forces that exist between molecules. When a carbon atom is bonded to another carbon atom, or to a hydrogen atom, the bond is not considered to be polar because the electronegativity difference between carbon atoms is zero and between carbon and ...
... 9. ANS: Dispersion forces are very weak intermolecular forces that exist between molecules. When a carbon atom is bonded to another carbon atom, or to a hydrogen atom, the bond is not considered to be polar because the electronegativity difference between carbon atoms is zero and between carbon and ...
Chemistry
... describe, in simple terms, the lattice structure of a crystalline solid which is: (i) ionic, as in sodium chloride, magnesium oxide (ii) simple molecular, as in iodine (iii) giant molecular, as in graphite; diamond (iv) hydrogen-bonded, as in ice (v) metallic, as in copper [the concept of the ‘unit ...
... describe, in simple terms, the lattice structure of a crystalline solid which is: (i) ionic, as in sodium chloride, magnesium oxide (ii) simple molecular, as in iodine (iii) giant molecular, as in graphite; diamond (iv) hydrogen-bonded, as in ice (v) metallic, as in copper [the concept of the ‘unit ...
Ground State and Bonding State Electronic Configurations
... Like valence-bond (VB) theory, molecular orbital (MO) theory recognizes that electrons cannot be localized on a single atom when that atom is part of a molecule. One major difference between the two methods is that MO theory calculates the molecular orbitals that may be produced from the available a ...
... Like valence-bond (VB) theory, molecular orbital (MO) theory recognizes that electrons cannot be localized on a single atom when that atom is part of a molecule. One major difference between the two methods is that MO theory calculates the molecular orbitals that may be produced from the available a ...
Investigating Matter Notes
... that can be ________________ or ________________. Characteristics that can be observed are called __________________ properties. Characteristics that can be measured are called __________________ properties. The set of properties that a particular substance has is unique. No other substance has that ...
... that can be ________________ or ________________. Characteristics that can be observed are called __________________ properties. Characteristics that can be measured are called __________________ properties. The set of properties that a particular substance has is unique. No other substance has that ...
2 Chemical bonding is a genuinely quantum effect, which cannot be
... This phenomenon will be discussed later on. A further important non-bonded contribution is the repulsion driven by Pauli exclusion principle. In contrast to the classical Coulomb interaction, it is of a purely quantummechanical origin. Two electrons with same spin try to avoid a large spatial overla ...
... This phenomenon will be discussed later on. A further important non-bonded contribution is the repulsion driven by Pauli exclusion principle. In contrast to the classical Coulomb interaction, it is of a purely quantummechanical origin. Two electrons with same spin try to avoid a large spatial overla ...
Chapter 18 Ketones and Aldehydes 1) Which of the following
... 97) Provide a detailed, stepwise mechanism for the acid-catalyzed condensation reaction between benzaldehyde and methylamine. 98) Conversion of aldehydes and ketones to imines is an acid-catalyzed process. Explain why this conversion is actually hindered by the presence of too much acid. 99) When H2 ...
... 97) Provide a detailed, stepwise mechanism for the acid-catalyzed condensation reaction between benzaldehyde and methylamine. 98) Conversion of aldehydes and ketones to imines is an acid-catalyzed process. Explain why this conversion is actually hindered by the presence of too much acid. 99) When H2 ...
Word - The Chemistry Book
... plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha particles (+), beta particles (-) and gamma rays (neutral). By performing other experiments and using this information, Rutherford created an atomic model different from Thoms ...
... plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha particles (+), beta particles (-) and gamma rays (neutral). By performing other experiments and using this information, Rutherford created an atomic model different from Thoms ...
SAMPL E PA GES
... Too much exposure to the sun’s UV radiation causes sunburn and the results – redness, blistered and peeling skin, and pain – are the same as the burns produced by intense heat. Repeated exposure to UV radiation over a longer period may produce skin cancers, especially among fair-haired, light-skinne ...
... Too much exposure to the sun’s UV radiation causes sunburn and the results – redness, blistered and peeling skin, and pain – are the same as the burns produced by intense heat. Repeated exposure to UV radiation over a longer period may produce skin cancers, especially among fair-haired, light-skinne ...