Describing Matter
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
Chemistry I
... 52. Increasing the temperature of a liquid solvent when dissolving a solid solute a. always increases the rate at which a solid solute dissolves b. often increases the amount of solid solute that can dissolve c. both a and b d. neither a and b ...
... 52. Increasing the temperature of a liquid solvent when dissolving a solid solute a. always increases the rate at which a solid solute dissolves b. often increases the amount of solid solute that can dissolve c. both a and b d. neither a and b ...
Carbon: The Building Blocks of Organic Compounds
... of these large molecules that are based around chains of carbon atoms. ...
... of these large molecules that are based around chains of carbon atoms. ...
Lecture 24 (Slides) October 18
... • 1. Which of the following atoms and ions are paramagnetic (i.e. have unpaired electrons). Note: An even number of electrons does not indicate that all electrons are paired. (a) He atom, (b) F atom, (c) As atom, (d) F- ion (e) Al3+ ion and (f) Fe atom. • 2. Arrange the following in order of increas ...
... • 1. Which of the following atoms and ions are paramagnetic (i.e. have unpaired electrons). Note: An even number of electrons does not indicate that all electrons are paired. (a) He atom, (b) F atom, (c) As atom, (d) F- ion (e) Al3+ ion and (f) Fe atom. • 2. Arrange the following in order of increas ...
Name_______________________ Answers to Final Exam Study
... when put into water. The reaction is very explosive. What group is this element most likely found in? a. ...
... when put into water. The reaction is very explosive. What group is this element most likely found in? a. ...
File
... is evidently true? A) The precision is poor, but the accuracy is excellent B) The precision is good, but the accuracy cannot be evaluated from the given information. C) The accuracy would be better if a more concentrated NaOH solution were used D) All three titrations have the same amount of error E ...
... is evidently true? A) The precision is poor, but the accuracy is excellent B) The precision is good, but the accuracy cannot be evaluated from the given information. C) The accuracy would be better if a more concentrated NaOH solution were used D) All three titrations have the same amount of error E ...
Working with Hazardous Chemicals
... Caution! Hexamethylphosphoric triamide (HMPA) vapors have been reported to cause cancer in rats.2 All operations with hexamethylphosphoric triamide should be performed in a good hood, and care should be taken to keep the liquid off the skin. Methyl iodide, in high concentrations for short periods or ...
... Caution! Hexamethylphosphoric triamide (HMPA) vapors have been reported to cause cancer in rats.2 All operations with hexamethylphosphoric triamide should be performed in a good hood, and care should be taken to keep the liquid off the skin. Methyl iodide, in high concentrations for short periods or ...
gorgpps.pps - Knockhardy
... All diagrams, photographs and any animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any work that is distributed for financial gain. ...
... All diagrams, photographs and any animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any work that is distributed for financial gain. ...
SAMPLE PAPER -4 Time Allowed: 3 Hrs
... Describe the principle involved in each of the following processes of metallurgy : (i) Froth floatation method (ii) Electrolytic refining of metals (iii) Zone refining of metals Q22 Give reasons for the following: a) H3PO3 is diprotic(dibasic). b) The electron gain enthalpy with negative sign for fl ...
... Describe the principle involved in each of the following processes of metallurgy : (i) Froth floatation method (ii) Electrolytic refining of metals (iii) Zone refining of metals Q22 Give reasons for the following: a) H3PO3 is diprotic(dibasic). b) The electron gain enthalpy with negative sign for fl ...
Stage 2 Chemistry Intended Student Learning 2014
... Explain the higher melting points and boiling points of polar substances compared with those of non-polar substances of similar molar mass. ...
... Explain the higher melting points and boiling points of polar substances compared with those of non-polar substances of similar molar mass. ...
Chapter 25 Organic and Biological Chemistry
... • The chain is numbered so the double bond gets the smallest possible number. • cis-Alkenes have the carbons in the chain on the same side of the molecule. • trans-Alkenes have the carbons in the chain on opposite sides of the molecule. ...
... • The chain is numbered so the double bond gets the smallest possible number. • cis-Alkenes have the carbons in the chain on the same side of the molecule. • trans-Alkenes have the carbons in the chain on opposite sides of the molecule. ...
stoichiometry - J. Seguin Science
... The proportions of reactants and products (the recipe) is referred to as the MOLE RATIO. ...
... The proportions of reactants and products (the recipe) is referred to as the MOLE RATIO. ...
3a-General Reactions 2010
... Any chemical reaction can be described as a molecular or atomic change. It produces one or more observable changes. e.g. color change, gas bubbles, heat, etc. Reactions are generally described as Reactant(s) --> Product(s) The reaction is written as a chemical equation with chemical formulas: 2 Na + ...
... Any chemical reaction can be described as a molecular or atomic change. It produces one or more observable changes. e.g. color change, gas bubbles, heat, etc. Reactions are generally described as Reactant(s) --> Product(s) The reaction is written as a chemical equation with chemical formulas: 2 Na + ...
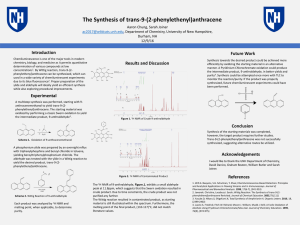
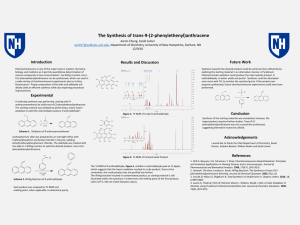
The Synthesis of trans-9-(2
... [email protected], Department of Chemistry, University of New Hampshire, Durham, NH ...
... [email protected], Department of Chemistry, University of New Hampshire, Durham, NH ...
IPC Semester Exam Review – Chemistry Topics
... 57. Draw atomic models for billiard ball through electron cloud. 58. Draw the Bohr model diagram for magnesium. 59. List the subatomic particles & isotope symbol for bromine-80. 60. Calculate the average atomic mass of lithium if 1 of 13 atoms is lithium-6 & the other 12 atoms are lithium-7. Chemica ...
... 57. Draw atomic models for billiard ball through electron cloud. 58. Draw the Bohr model diagram for magnesium. 59. List the subatomic particles & isotope symbol for bromine-80. 60. Calculate the average atomic mass of lithium if 1 of 13 atoms is lithium-6 & the other 12 atoms are lithium-7. Chemica ...
+1/2
... The coupling constant is the distance J (measured in Hz) between the peaks in a multiplet. J is a measure of the amount of interaction between the two sets of hydrogens creating the multiplet. ...
... The coupling constant is the distance J (measured in Hz) between the peaks in a multiplet. J is a measure of the amount of interaction between the two sets of hydrogens creating the multiplet. ...