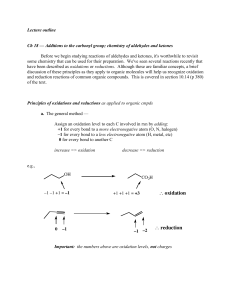
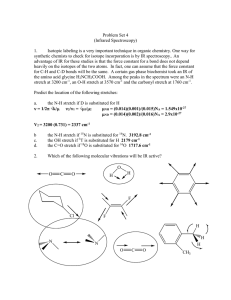
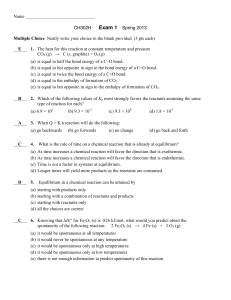
2.5 Chemical Bonding - Lighthouse Christian Academy
... • Oppositely charged ions (metals and non-metals) have a strong attraction for one another and, as a result, are held tightly together. • This is known as ionic bonding and serves to build atoms into compounds called ionic compounds. • In ionic bonding, a transfer of valence e occurs. • Ionic bondi ...
... • Oppositely charged ions (metals and non-metals) have a strong attraction for one another and, as a result, are held tightly together. • This is known as ionic bonding and serves to build atoms into compounds called ionic compounds. • In ionic bonding, a transfer of valence e occurs. • Ionic bondi ...
Unit 2 (Biochemistry) Review
... You should be able to tell the difference between ions and atoms, and be able to determine what type of ion is present. 3) I can compare the types of bonding between atoms to form molecules. (A.5.c) Ionic Bond Covalent Bond Metallic Bond Charge Valence Electron Oxidation Number You should be able to ...
... You should be able to tell the difference between ions and atoms, and be able to determine what type of ion is present. 3) I can compare the types of bonding between atoms to form molecules. (A.5.c) Ionic Bond Covalent Bond Metallic Bond Charge Valence Electron Oxidation Number You should be able to ...
Energetics of the primary electron transfer reaction revealed by
... transfer (ET) reactions along the chromophore chain (see inset Fig. 1) in photosynthetic reaction centers (RCs) leads to a reduction of the quinone QA within = 200 ps [ 14 1. The preceding intermediate, the radical pair state P+Hz , where the electron resides on the bacteriopheophytin HA, is formed ...
... transfer (ET) reactions along the chromophore chain (see inset Fig. 1) in photosynthetic reaction centers (RCs) leads to a reduction of the quinone QA within = 200 ps [ 14 1. The preceding intermediate, the radical pair state P+Hz , where the electron resides on the bacteriopheophytin HA, is formed ...
L-13
... of allylmagnesium chloride), which is a typical highly nucleophilic reagent, instead of 1, but the starting alcohol was recovered after work-up under conditions with or without the InCl3 catalyst. These results strongly suggest that the appropriate nucleophilicity of the allylic reagent and Lewis ac ...
... of allylmagnesium chloride), which is a typical highly nucleophilic reagent, instead of 1, but the starting alcohol was recovered after work-up under conditions with or without the InCl3 catalyst. These results strongly suggest that the appropriate nucleophilicity of the allylic reagent and Lewis ac ...
Ch.08An Introduction to Metabolism
... • More free energy (higher G) • Less stable • Greater work capacity ...
... • More free energy (higher G) • Less stable • Greater work capacity ...
Original
... Electrostatic attraction between closely packed, oppositely charged metal and nonmetal ions, form ionic bonds. The energy of ionic bonds can be calculated using Coulomb’s Law, where Q = the charge of each ion, and r = distance between ions (nm): (Negative answer = attraction, positive answer = repul ...
... Electrostatic attraction between closely packed, oppositely charged metal and nonmetal ions, form ionic bonds. The energy of ionic bonds can be calculated using Coulomb’s Law, where Q = the charge of each ion, and r = distance between ions (nm): (Negative answer = attraction, positive answer = repul ...
Equilibrium Constant
... This specific reaction simplifies matters to the point that we can consider the activities of the products when determining Ksp. We would call this the activity product [AP] or ion activity product if only charged species are involved [IAP]. If the [AP] = Ksp, the system is in equilibrium,. If the [ ...
... This specific reaction simplifies matters to the point that we can consider the activities of the products when determining Ksp. We would call this the activity product [AP] or ion activity product if only charged species are involved [IAP]. If the [AP] = Ksp, the system is in equilibrium,. If the [ ...
June 2011 review
... Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule. [1] 9. Base your answer on the information below. In 1864, the Solvay process was developed to make soda ...
... Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule. [1] 9. Base your answer on the information below. In 1864, the Solvay process was developed to make soda ...
Chemistry in Society - Cathkin High School
... They must take account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. ...
... They must take account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. ...
CHEM 2414
... 1. For a monosubstituted cycloalkane the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary. 2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substi ...
... 1. For a monosubstituted cycloalkane the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary. 2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substi ...
Yeast Reduction #812
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
... Treatment of a ketone with either agent will reduce the carbonyl group to yield an alcohol. However, these reducing agents do not react to form a chiral alcohol because the hydride can attack both sides of the planar carbonyl group yielding a mixture of both enantiomers.4 In order to achieve a chira ...
Unit F322 - Chains, energy and resources - Visually impaired
... structure fits with the information given above. __________________________________________ __________________________________________ __________________________________________ __________________________________________ __________________________________________ ____________________________________ ...
... structure fits with the information given above. __________________________________________ __________________________________________ __________________________________________ __________________________________________ __________________________________________ ____________________________________ ...
Lecture 10 Activity of chemical components
... Since it is like 1:1 salt, the solution activity coefficient is less than one. Hence the apparent rate constant is modified and it is lesser than the one measured in very dilute solution. For example in 0.1 molar solutions it could differ by factor of three. Similarly, if we were to consider the eff ...
... Since it is like 1:1 salt, the solution activity coefficient is less than one. Hence the apparent rate constant is modified and it is lesser than the one measured in very dilute solution. For example in 0.1 molar solutions it could differ by factor of three. Similarly, if we were to consider the eff ...
Webquest Review - Harrison High School
... polar and will have dipole-dipole interactions between molecules. That means CH3Cl will be harder to boil than CO2. H2O has H bonded to O and it’s polar. When you have species with H bonded to F, O, or N, hydrogen “bond”ing sets up between the molecules. Hydrogen bonding is the strongest intermolecu ...
... polar and will have dipole-dipole interactions between molecules. That means CH3Cl will be harder to boil than CO2. H2O has H bonded to O and it’s polar. When you have species with H bonded to F, O, or N, hydrogen “bond”ing sets up between the molecules. Hydrogen bonding is the strongest intermolecu ...
Health and Safety Services
... 16. If thermal runaway is a possibility, the risk assessment must address all reasonably practicable means of eliminating the risk though process controls, but will probably also have to specify precautionary measures to minimise the damage caused by such an event. These might include: ...
... 16. If thermal runaway is a possibility, the risk assessment must address all reasonably practicable means of eliminating the risk though process controls, but will probably also have to specify precautionary measures to minimise the damage caused by such an event. These might include: ...
Properties and Changes in Matter
... Elements, Compounds, and Mixtures Elements are pure substances composed of only one type of matter Compounds are substances made up of more than one type of matter acting like a single pure substance. Mixtures are made up of more than one substance in which each part Retains its chemical identity. ...
... Elements, Compounds, and Mixtures Elements are pure substances composed of only one type of matter Compounds are substances made up of more than one type of matter acting like a single pure substance. Mixtures are made up of more than one substance in which each part Retains its chemical identity. ...
Chemistry 520 - Problem Set 2
... 3. A bomb calorimeter provides a way to measure qV for a reaction of interest by constraining it to take place in a closed vessel which is surrounded by a insulated container of water. A gure depicting a bomb calorimeter is attached. In a typical experiment, one rst calibrates the calorimeter by r ...
... 3. A bomb calorimeter provides a way to measure qV for a reaction of interest by constraining it to take place in a closed vessel which is surrounded by a insulated container of water. A gure depicting a bomb calorimeter is attached. In a typical experiment, one rst calibrates the calorimeter by r ...