Chairman, OJEE-2015
... of cooling. Kinetic Theory of gases- Pressure of an ideal gas, Kinetic interpretation of temperature, Degrees of freedom, Law of equipartition of energy. First Law of Thermodynamics, Specific heats of a gaseous system, Relation between Cp and Cv, Work done during Isothermal and Adiabatic processes, ...
... of cooling. Kinetic Theory of gases- Pressure of an ideal gas, Kinetic interpretation of temperature, Degrees of freedom, Law of equipartition of energy. First Law of Thermodynamics, Specific heats of a gaseous system, Relation between Cp and Cv, Work done during Isothermal and Adiabatic processes, ...
User Guide - OJEE 2015
... of cooling. Kinetic Theory of gases- Pressure of an ideal gas, Kinetic interpretation of temperature, Degrees of freedom, Law of equipartition of energy. First Law of Thermodynamics, Specific heats of a gaseous system, Relation between Cp and Cv, Work done during Isothermal and Adiabatic processes, ...
... of cooling. Kinetic Theory of gases- Pressure of an ideal gas, Kinetic interpretation of temperature, Degrees of freedom, Law of equipartition of energy. First Law of Thermodynamics, Specific heats of a gaseous system, Relation between Cp and Cv, Work done during Isothermal and Adiabatic processes, ...
CH_12_3_Reactions_Alcohols_Thiols
... Select the product when CH3–CH2–CH2–OH undergoes each of the following reactions: ...
... Select the product when CH3–CH2–CH2–OH undergoes each of the following reactions: ...
Chapter 2 Chemistry
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
Partial Pressures of Gases
... solution of silver nitrate, AgNO3(aq). The solution gradually changes colour to blue, and sparkling crystals form on the copper wire. Diagnostic tests confirm that the blue solution is due to formation of copper(II) nitrate, Cu(NO3)2(aq), which goes into solution, and the crystals are metallic silve ...
... solution of silver nitrate, AgNO3(aq). The solution gradually changes colour to blue, and sparkling crystals form on the copper wire. Diagnostic tests confirm that the blue solution is due to formation of copper(II) nitrate, Cu(NO3)2(aq), which goes into solution, and the crystals are metallic silve ...
Unit3_Notes - Lesmahagow High School
... account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. Atom Economy Atom economy is a measure of the proportion of reactant atoms which are incorp ...
... account of cost of raw materials, plant-running costs etc. If the yield of product is not sufficient enough to cover the costs of production then the process would not be considered to be economically viable. Atom Economy Atom economy is a measure of the proportion of reactant atoms which are incorp ...
Semester 2 Final Exam
... energy, its temperature increases from 10°C to 47°C. What is the mass of this block? (c of Al = 0.900 J/g·°C) (A) 0.055 g (B) 14.6 g (C) 18.0 g (D) 33.3 g 6. The units for heat are: (A) J (B) J/g (C) J/g·°C (D) J/°C 7. 20.0 gram samples of each of the following metals are originally at 10°C. They ar ...
... energy, its temperature increases from 10°C to 47°C. What is the mass of this block? (c of Al = 0.900 J/g·°C) (A) 0.055 g (B) 14.6 g (C) 18.0 g (D) 33.3 g 6. The units for heat are: (A) J (B) J/g (C) J/g·°C (D) J/°C 7. 20.0 gram samples of each of the following metals are originally at 10°C. They ar ...
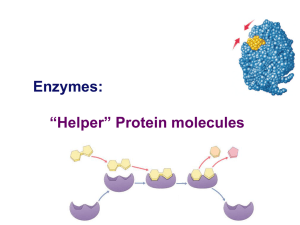
Enzymes: “Helper” Protein molecules
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
... Enzymes aren’t used up Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
FINAL EXAM Spring 2012
... E) Ksp = [Au3+][3OH-]3 20) Z- is a weak base. An aqueous solution of NaZ is prepared by dissolving 0.350 mol of NaZ insufficient water to yield 1.0 L of solution. The pH of the solution was 8.93 at 25.0 °C. The Kb of Z- is __________. A) 6.9 × 10-9 B) 2.1 × 10-10 C) 2.8 × 10-12 D) 9.9 × 10-2 E) 1.2 ...
... E) Ksp = [Au3+][3OH-]3 20) Z- is a weak base. An aqueous solution of NaZ is prepared by dissolving 0.350 mol of NaZ insufficient water to yield 1.0 L of solution. The pH of the solution was 8.93 at 25.0 °C. The Kb of Z- is __________. A) 6.9 × 10-9 B) 2.1 × 10-10 C) 2.8 × 10-12 D) 9.9 × 10-2 E) 1.2 ...
AP® Chemistry 2009 Free-Response Questions - AP Central
... (d) For reaction Y at 298 K, which is larger: the total bond energy of the reactants or the total bond energy of the products? Explain. (e) Is the following statement true or false? Justify your answer. “On the basis of the data in the table, it can be predicted that reaction Y will occur more rapid ...
... (d) For reaction Y at 298 K, which is larger: the total bond energy of the reactants or the total bond energy of the products? Explain. (e) Is the following statement true or false? Justify your answer. “On the basis of the data in the table, it can be predicted that reaction Y will occur more rapid ...
Name - Quia
... Explain why nuclear reactions occur and know how to balance a nuclear equation. Define and relate the terms radioactive decay and nuclear radiation. Describe the different types of radioactive decay and their effects on the nucleus. Define the term half-life, and explain how it relates to the stabil ...
... Explain why nuclear reactions occur and know how to balance a nuclear equation. Define and relate the terms radioactive decay and nuclear radiation. Describe the different types of radioactive decay and their effects on the nucleus. Define the term half-life, and explain how it relates to the stabil ...
Section (c) – The Structure of atoms
... 13. An electric current in a ……………….…… is a flow of electrons. All metals and the non-metal element …………………………………………………. conduct electricity. In a d.c. supply the electrons flow from the …………………………………………………. electrode to the …………………………………………………. electrode. Metal compounds do not conduct in the …………… ...
... 13. An electric current in a ……………….…… is a flow of electrons. All metals and the non-metal element …………………………………………………. conduct electricity. In a d.c. supply the electrons flow from the …………………………………………………. electrode to the …………………………………………………. electrode. Metal compounds do not conduct in the …………… ...
Phy 211: General Physics I
... A pure substance cannot be broken down into its component substances by physical means only by a chemical process 1. The breakdown of a pure substance results in formation of new substances (i.e. chemical change) 2. For a pure substance there is nothing to separate (its only 1 substance to begin wit ...
... A pure substance cannot be broken down into its component substances by physical means only by a chemical process 1. The breakdown of a pure substance results in formation of new substances (i.e. chemical change) 2. For a pure substance there is nothing to separate (its only 1 substance to begin wit ...
D. Hydrogen bonds
... a) Oxygen and nitrogen are more electronegative than hydrogen (1) When hydrogen is covalently bound to O or N, the hydrogen will have a partial positive charge (2) It will therefore be attracted to atoms with slightly negative charges, usually another O or N E. Bonds in proteins and nucleic acids 1. ...
... a) Oxygen and nitrogen are more electronegative than hydrogen (1) When hydrogen is covalently bound to O or N, the hydrogen will have a partial positive charge (2) It will therefore be attracted to atoms with slightly negative charges, usually another O or N E. Bonds in proteins and nucleic acids 1. ...
Topic 5 - Chemical Reactions
... Matter can be described using both chemical and physical properties. Elements are the simplest form of matter that has a unique set of properties. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. 4. Chemical formulas are used to represent compou ...
... Matter can be described using both chemical and physical properties. Elements are the simplest form of matter that has a unique set of properties. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. 4. Chemical formulas are used to represent compou ...
polymerisation
... temperature and a catalyst. The catalyst is usually a substance (organic peroxide) which readily breaks up to form radicals which, in turn, initiate a chain reaction. Another famous type of catalyst is a Ziegler-Natta catalyst (named after the scientists who developed it). Such catalysts are based o ...
... temperature and a catalyst. The catalyst is usually a substance (organic peroxide) which readily breaks up to form radicals which, in turn, initiate a chain reaction. Another famous type of catalyst is a Ziegler-Natta catalyst (named after the scientists who developed it). Such catalysts are based o ...
ALCOHOLS
... the human food chain is now being used to grow sugar to make biofuels. However, ethanol produced in this way is considered to be carbon neutral since all of the CO2 released during its combustion was removed during photosynthesis by the growing crop. It can not be considered to be 100% carbon neutra ...
... the human food chain is now being used to grow sugar to make biofuels. However, ethanol produced in this way is considered to be carbon neutral since all of the CO2 released during its combustion was removed during photosynthesis by the growing crop. It can not be considered to be 100% carbon neutra ...
Basic Chemistry – Terminology and Reactions
... An activity series is a list of substances ranked in order of relative reactivity. For example, magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental hydrogen: Mg (s) + 2 HCl (aq) ...
... An activity series is a list of substances ranked in order of relative reactivity. For example, magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental hydrogen: Mg (s) + 2 HCl (aq) ...
Chemistry
... of matter that gives rise to these interactions. At O-Level, students have been introduced to the fundamental idea that matter is made up of particles and the simple atomic model (electrons in discrete shells around a positively charged nucleus). This allows students to apply the key ideas of conser ...
... of matter that gives rise to these interactions. At O-Level, students have been introduced to the fundamental idea that matter is made up of particles and the simple atomic model (electrons in discrete shells around a positively charged nucleus). This allows students to apply the key ideas of conser ...
Stoichiometry
... Two compounds are involved with the cation of one compound EXCHANGING with the cation of another compound. AX + BZ AZ + BX These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The ...
... Two compounds are involved with the cation of one compound EXCHANGING with the cation of another compound. AX + BZ AZ + BX These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The ...
lect2_htm
... why chemical reactions have a barrier. The Rule applies to molecular orbitals as well as wavefunctions for whole molecules. For example, consider a situation where a molecule has two molecular orbitals. The energies of these MOs are altered by a geometric change in the molecule Each MO can be catego ...
... why chemical reactions have a barrier. The Rule applies to molecular orbitals as well as wavefunctions for whole molecules. For example, consider a situation where a molecule has two molecular orbitals. The energies of these MOs are altered by a geometric change in the molecule Each MO can be catego ...