Types of Chemical Reactions
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
... Thousands of known chemical reactions occur in various systems. Memorizing the equations for so many chemical reactions would be difficult. It is more useful and realistic to classify reactions according to various similarities and regularities. ...
chemistry- sch4u - final exam
... e. all of the above depending on the reaction In the reaction, N2(g) + O2(g) 2NO2(g) , if the concentration changes from 0.45 mol/L to 1.00 mol/L in 2 minutes, what is the overall rate of production of nitrogen dioxide in the system? a. 3.64 mol/(L·min) d. 12.6 mol/(L·min) b. 0.275 mol/(L·min) e. ...
... e. all of the above depending on the reaction In the reaction, N2(g) + O2(g) 2NO2(g) , if the concentration changes from 0.45 mol/L to 1.00 mol/L in 2 minutes, what is the overall rate of production of nitrogen dioxide in the system? a. 3.64 mol/(L·min) d. 12.6 mol/(L·min) b. 0.275 mol/(L·min) e. ...
∙ ∙B x
... 1. Draw the structural formula of water showing its shape. 2. What are the electronegativites of oxygen and hydrogen? 3. Are the bonding electrons shared equally between oxygen and hydrogen? 4. Where is the highest probability of finding them? 5. Is there an even distribution of bonding electrons in ...
... 1. Draw the structural formula of water showing its shape. 2. What are the electronegativites of oxygen and hydrogen? 3. Are the bonding electrons shared equally between oxygen and hydrogen? 4. Where is the highest probability of finding them? 5. Is there an even distribution of bonding electrons in ...
∙ ∙B x
... 1. Draw the structural formula of water showing its shape. 2. What are the electronegativites of oxygen and hydrogen? 3. Are the bonding electrons shared equally between oxygen and hydrogen? 4. Where is the highest probability of finding them? 5. Is there an even distribution of bonding electrons in ...
... 1. Draw the structural formula of water showing its shape. 2. What are the electronegativites of oxygen and hydrogen? 3. Are the bonding electrons shared equally between oxygen and hydrogen? 4. Where is the highest probability of finding them? 5. Is there an even distribution of bonding electrons in ...
Carbon-12 Stable
... Entropy- disorder or lack of organizational structure between molecules -Entropy usually increases as energy increases -High entropy means matter cannon take a definite shape, low entropy allows for a definite shape What is easier to control, a group of tired, well-behaved kids or a group of ...
... Entropy- disorder or lack of organizational structure between molecules -Entropy usually increases as energy increases -High entropy means matter cannon take a definite shape, low entropy allows for a definite shape What is easier to control, a group of tired, well-behaved kids or a group of ...
Chapter 3: Introduction to chemical formulas and reactivity
... 2. Fill out remaining elements, changing coefficients as needed, until the number of each type of atom is same on both sides of the equation. ...
... 2. Fill out remaining elements, changing coefficients as needed, until the number of each type of atom is same on both sides of the equation. ...
Document
... STA: S.11.C.1.1.2 TOP: Describe the structures of alkanes. KEY: Structure and formula of alkane MSC: 2 15. ANS: a Butane is used as a domestic fuel because it readily undergoes combustion in oxygen to produce a huge quantity of heat and energy. b Butane does not dissolve in water because water is a ...
... STA: S.11.C.1.1.2 TOP: Describe the structures of alkanes. KEY: Structure and formula of alkane MSC: 2 15. ANS: a Butane is used as a domestic fuel because it readily undergoes combustion in oxygen to produce a huge quantity of heat and energy. b Butane does not dissolve in water because water is a ...
Document
... Alcohols possess the OH group and so can form hydrogen bonds. Alcohols are soluble in water because they are capable of forming hydrogen bonds with water molecules. Other organic compounds, which are not able to form hydrogen bonds do not dissolve in water. Halogenoalkanes for example, although pola ...
... Alcohols possess the OH group and so can form hydrogen bonds. Alcohols are soluble in water because they are capable of forming hydrogen bonds with water molecules. Other organic compounds, which are not able to form hydrogen bonds do not dissolve in water. Halogenoalkanes for example, although pola ...
kinetics and equilibrium
... – Pollution SO2, CO2, NO • Converter lets H2O react w/ gases to convert them to weak acids (more complete combustion) ...
... – Pollution SO2, CO2, NO • Converter lets H2O react w/ gases to convert them to weak acids (more complete combustion) ...
ψ 2
... photon will be given approximately by the difference in the energies of the excited orbital and the 1sg ground state orbital. Thus molecules as well as atoms will exhibit a line spectrum. The electronic line spectrum obtained from a molecule is, however, complicated by the appearance of many accompa ...
... photon will be given approximately by the difference in the energies of the excited orbital and the 1sg ground state orbital. Thus molecules as well as atoms will exhibit a line spectrum. The electronic line spectrum obtained from a molecule is, however, complicated by the appearance of many accompa ...
Part I - American Chemical Society
... Part I of this test is designed to be taken with a Scantron® answer sheet on which the student records his or her responses. Only this Scantron sheet is graded for a score on Part I. Testing materials, scratch paper, and the Scantron sheet should be made available to the student only during the exam ...
... Part I of this test is designed to be taken with a Scantron® answer sheet on which the student records his or her responses. Only this Scantron sheet is graded for a score on Part I. Testing materials, scratch paper, and the Scantron sheet should be made available to the student only during the exam ...
Gateway Chemistry Review (Answer Key) Structure and Properties
... o The ability of an atom to attract and hold extra electrons. Electronegativity o The tendency of an atom to attract electrons to itself when combined with another atom. Ionization energy o Amount of energy required to remove an electron from an atom or ion. Atomic Radius o One half the distan ...
... o The ability of an atom to attract and hold extra electrons. Electronegativity o The tendency of an atom to attract electrons to itself when combined with another atom. Ionization energy o Amount of energy required to remove an electron from an atom or ion. Atomic Radius o One half the distan ...
Reaction Stoichiometry
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
... We cannot simply add the total moles of all the reactants to decide which reactant mixture makes the most product. We must always think about how much product can be formed by using what we are given, and the ratio in the balanced equation. ...
Chapter 1 - TamAPChemistryHart
... 15. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solid ...
... 15. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solid ...
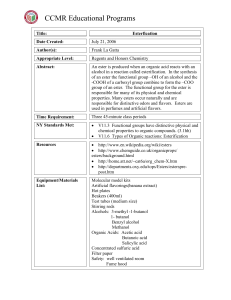
Esterification
... Pre- Lab Discussion: Esters are responsible for the smell of many fruits and perfumes. In this experiment, a microscale technique is used to prepare four different esters. The esters are identified by their odors. Objectives: Students will use qualitative skills to determine the esters formed in thi ...
... Pre- Lab Discussion: Esters are responsible for the smell of many fruits and perfumes. In this experiment, a microscale technique is used to prepare four different esters. The esters are identified by their odors. Objectives: Students will use qualitative skills to determine the esters formed in thi ...
Lecture4_Ch3_091905!
... (3.2) The Periodic Table Classical example of the building of a paradigm: Repeated patterns of similarity in the composition of binary compounds triggered a search for order and organization of the elements in terms of observable properties. First organization of the periodic: By atomic mass. Perio ...
... (3.2) The Periodic Table Classical example of the building of a paradigm: Repeated patterns of similarity in the composition of binary compounds triggered a search for order and organization of the elements in terms of observable properties. First organization of the periodic: By atomic mass. Perio ...
Lecture 4
... (1) The elements can be arranged in groups (columns) of elements that possess related chemical and physical properties. (2) The elements can be arranged in periods (rows) of elements that possess progressively different physical and chemical properties. (3) Original Paradigm: The chemical and physic ...
... (1) The elements can be arranged in groups (columns) of elements that possess related chemical and physical properties. (2) The elements can be arranged in periods (rows) of elements that possess progressively different physical and chemical properties. (3) Original Paradigm: The chemical and physic ...
02Ch02chemistry2005
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
Hess`s law and Bond Enthalpy Practice
... a) Calculate Δ Ho for the decomposition reaction. b) Calculate q for the reaction when 1.00 kg of NH4NO3 decomposes. 3) One might think that we could react SO2 (g) with oxygen to produce SO3 (g), but this reaction does not occur readily. Instead, the following two step sequence may be used to produc ...
... a) Calculate Δ Ho for the decomposition reaction. b) Calculate q for the reaction when 1.00 kg of NH4NO3 decomposes. 3) One might think that we could react SO2 (g) with oxygen to produce SO3 (g), but this reaction does not occur readily. Instead, the following two step sequence may be used to produc ...
Chapter 4 Knowledge Check
... Identify the choice that best completes the statement or answers the question. 53. Organic chemistry is a science based on the study of a. functional groups. b. vital forces interacting with matter. c. carbon compounds. d. water and its interaction with other kinds of molecules. e. inorganic compoun ...
... Identify the choice that best completes the statement or answers the question. 53. Organic chemistry is a science based on the study of a. functional groups. b. vital forces interacting with matter. c. carbon compounds. d. water and its interaction with other kinds of molecules. e. inorganic compoun ...