... As the need for larger quantities of the catalyst grew, more efficient methods for its synthesis were required. A practical route to ruthenium benzylidene complexes was developed. In 1995 Grubbs reported new molecularly-well-defined catalysts [Ru(=CHPh)Cl2(PR3)2], R = Ph or Cy (cyclohexyl).8g,h Thes ...
Many Chemistries Could Be Used to Build Living Systems
... single bonds with many other non-metals, and double and triple bonds with itself and a few other, non-metallic elements, forming complex functional groups that can bond to all other elements (with the possible exception of the noble gases). Carbon can also form polymers, bonded to itself or to other ...
... single bonds with many other non-metals, and double and triple bonds with itself and a few other, non-metallic elements, forming complex functional groups that can bond to all other elements (with the possible exception of the noble gases). Carbon can also form polymers, bonded to itself or to other ...
Solution Preparation Final Goueth
... (D) The average velocity of the O2 molecules is one-half that of the CH4 molecules. 65. 168.00 J of energy are added to a sample of gallium initially at 25.0 °C, the temperature rises to 38.0 °C. What is the volume of the sample? Data for Gallium, Ga specific heat 0.372 J g¯1 °C¯1 ...
... (D) The average velocity of the O2 molecules is one-half that of the CH4 molecules. 65. 168.00 J of energy are added to a sample of gallium initially at 25.0 °C, the temperature rises to 38.0 °C. What is the volume of the sample? Data for Gallium, Ga specific heat 0.372 J g¯1 °C¯1 ...
Practice Problem Set #6
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
The characteristic reaction of aromatic rings
... Reactions of the Side Chain of Alkylbenzenes t Benzylic Radicals and Cations l When toluene undergoes hydrogen abstraction from its methyl group it produces a benzyl radical è A benzylic radical is a radical in which the carbon bearing the unpaired electron is directly bonded to an aromatic ring ...
... Reactions of the Side Chain of Alkylbenzenes t Benzylic Radicals and Cations l When toluene undergoes hydrogen abstraction from its methyl group it produces a benzyl radical è A benzylic radical is a radical in which the carbon bearing the unpaired electron is directly bonded to an aromatic ring ...
Introductory Review
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
... For ionic compounds, e.g. sodium chloride, the formula shows the ratio of elements that form the compound. Solid sodium chloride consists of a collection of positively charged sodium ions and negatively charged chloride ions in a three-dimensional structure. You cannot say which sodium ion is assoc ...
WORD - SSS Chemistry
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
1.8 M - Thierry Karsenti
... and export them as consistently styled content within Learning Objects. This frees the content developer to focus on the quality of the content without having to overly concern themselves with presentation. Similarly, editors of learning objects need not concern themselves with ensuring authors use ...
... and export them as consistently styled content within Learning Objects. This frees the content developer to focus on the quality of the content without having to overly concern themselves with presentation. Similarly, editors of learning objects need not concern themselves with ensuring authors use ...
ECE final exam_fall 2013
... _________ 1. A molecule that follows the octet rule has a central atom and 4 surrounding atoms. The geometry must be tetrahedral. _________ 2. A photon’s energy is directly proportional to its wavelength. _________ 3. In the hydrogen atom a transition from n = 2 to n = 3 requires the same amount of ...
... _________ 1. A molecule that follows the octet rule has a central atom and 4 surrounding atoms. The geometry must be tetrahedral. _________ 2. A photon’s energy is directly proportional to its wavelength. _________ 3. In the hydrogen atom a transition from n = 2 to n = 3 requires the same amount of ...
3. Organic Compounds: Alkanes and
... close to the axial hydrogens three carbons away on C3 and C5, resulting in 7.6 kJ/mol of steric strain ...
... close to the axial hydrogens three carbons away on C3 and C5, resulting in 7.6 kJ/mol of steric strain ...
N2(g)
... – the study of transformations of energy – especially as heat and work Used to describe mechanical systems e.g. steam engine all chemical processes (e.g. combustion, dissolving of a solid, expansion of a gas) also involve exchange of heat or work. Terms you’ll use in thermodynamics: Energy – the cap ...
... – the study of transformations of energy – especially as heat and work Used to describe mechanical systems e.g. steam engine all chemical processes (e.g. combustion, dissolving of a solid, expansion of a gas) also involve exchange of heat or work. Terms you’ll use in thermodynamics: Energy – the cap ...
Communicating Research to the General Public
... reactions they perform. 8.2.1. Atoms are made of protons, neutrons, and electrons. Atoms consist of some number and arrangement of three sub-atomic particles called protons, neutrons, and electrons. These particles differ in three significant ways. First, in the electrical charge they carry: protons ...
... reactions they perform. 8.2.1. Atoms are made of protons, neutrons, and electrons. Atoms consist of some number and arrangement of three sub-atomic particles called protons, neutrons, and electrons. These particles differ in three significant ways. First, in the electrical charge they carry: protons ...
Aldehydes and Ketones
... But how does a nitrile group behave? What could be happening? We are breaking the CN bond; bond order goes from 3 to 0. Probably stepwise. Chemically speaking: the nitrogen of the nitrile is basic (lone pair) and can be protonated. This makes it a better electrophile (cf. carbonyl). Multiple bond ca ...
... But how does a nitrile group behave? What could be happening? We are breaking the CN bond; bond order goes from 3 to 0. Probably stepwise. Chemically speaking: the nitrogen of the nitrile is basic (lone pair) and can be protonated. This makes it a better electrophile (cf. carbonyl). Multiple bond ca ...
Unit D: Quantitative Relationships in Chemical Change
... Every chemical reaction involves the rearrangement of atoms into different combinations. However, during these reactions, the total number of atoms of each type of element is the same after the reaction as it was before the reaction. ...
... Every chemical reaction involves the rearrangement of atoms into different combinations. However, during these reactions, the total number of atoms of each type of element is the same after the reaction as it was before the reaction. ...
Chemistry - cloudfront.net
... 42. given a Lewis structure, be able to compute the formal charge for an atom 43. given a covalent bond between two atoms, be able to discern if the bond is polar or non-polar 44. given a molecular formula, be able to draw the Lewis structure to be able to tell if the molecule is polar or non-polar ...
... 42. given a Lewis structure, be able to compute the formal charge for an atom 43. given a covalent bond between two atoms, be able to discern if the bond is polar or non-polar 44. given a molecular formula, be able to draw the Lewis structure to be able to tell if the molecule is polar or non-polar ...
Solution Stoichiometry - Angelo State University
... • For a chemical reaction to occur, the reacting species have to come in close contact with each other. Most chemical reactions are performed in a solution (or in the gas phase) rather than in the solid state. • A solution consists of a smaller amount of one substance, the solute (usually a liquid o ...
... • For a chemical reaction to occur, the reacting species have to come in close contact with each other. Most chemical reactions are performed in a solution (or in the gas phase) rather than in the solid state. • A solution consists of a smaller amount of one substance, the solute (usually a liquid o ...
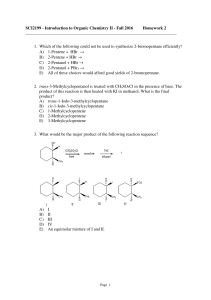
SCI2199 - Introduction to Organic Chemistry II
... through use of which of these reagents? A) Concd. HCl B) SO2Cl2 C) NaCl, H2SO4 D) PCl3 E) POCl3 29. Which of the following could be used to synthesize 2-iodobutane? A) CH3CH2CH=CH2 + I2(aq) → B) CH3CH2CHOHCH3 + HI → C) CH3CH2C≡CH + HI → D) CH3CH2C≡CH + I2 → E) None of these choices. ...
... through use of which of these reagents? A) Concd. HCl B) SO2Cl2 C) NaCl, H2SO4 D) PCl3 E) POCl3 29. Which of the following could be used to synthesize 2-iodobutane? A) CH3CH2CH=CH2 + I2(aq) → B) CH3CH2CHOHCH3 + HI → C) CH3CH2C≡CH + HI → D) CH3CH2C≡CH + I2 → E) None of these choices. ...
AP Chemistry: Total Notes Review
... 3: Fill the octets 4: use double and triple bonds as necessary o Formal charges: subtract the amount of electrons on the periodic table (for that element) from the electrons you drew in ~ 0 means right on ~ the negative charge should be on the most electronegative atom o Resonance: when one Lewis st ...
... 3: Fill the octets 4: use double and triple bonds as necessary o Formal charges: subtract the amount of electrons on the periodic table (for that element) from the electrons you drew in ~ 0 means right on ~ the negative charge should be on the most electronegative atom o Resonance: when one Lewis st ...