C H
... in the molecule. The ability to form two or more molecules with different configuration is called stereoisomerism. Stereocenter is defined as an atom bearing groups such that an interchanging of any two groups leads to a stereoisomer. A tetrahedral atom with four different groups attached to it is a ...
... in the molecule. The ability to form two or more molecules with different configuration is called stereoisomerism. Stereocenter is defined as an atom bearing groups such that an interchanging of any two groups leads to a stereoisomer. A tetrahedral atom with four different groups attached to it is a ...
AP Chemistry
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...
Chemical Thermodynamics presentation 1
... Because ΔG° is positive, the reaction is not spontaneous under standard conditions at 298 K. Note: We had to convert the units of the T ΔS term to kJ sp that it could be added to the ΔH term whose units are kJ ...
... Because ΔG° is positive, the reaction is not spontaneous under standard conditions at 298 K. Note: We had to convert the units of the T ΔS term to kJ sp that it could be added to the ΔH term whose units are kJ ...
Equilibrium Review Problems N2(g) + H2(g) NH3(g) 1. When 3.29
... In a saturated solution of MgF2 at 18ºC, the concentration of Mg2+ is 1.2110-3 molar. The equilibrium is represented by the equation above. (a) Write the expression for the solubility-product constant, Ksp, and calculate its value at 18ºC. (b) Calculate the equilibrium concentration of Mg2+ in 1.00 ...
... In a saturated solution of MgF2 at 18ºC, the concentration of Mg2+ is 1.2110-3 molar. The equilibrium is represented by the equation above. (a) Write the expression for the solubility-product constant, Ksp, and calculate its value at 18ºC. (b) Calculate the equilibrium concentration of Mg2+ in 1.00 ...
Now we turn to the study of chemical kinetics. Kinetics is the study of
... species changes with time. We are interested in this because it allows us to know before we run a reaction how long it will take for a reactant to be 90% consumed or half consumed. In order to do this we are going to determine equations that relate the concentration of a reactant with time. This inv ...
... species changes with time. We are interested in this because it allows us to know before we run a reaction how long it will take for a reactant to be 90% consumed or half consumed. In order to do this we are going to determine equations that relate the concentration of a reactant with time. This inv ...
L22 - Supplementary Student Notes Package
... Every chemical reaction involves the rearrangement of atoms into different combinations. However, during these reactions, the total number of atoms of each type of element is the same after the reaction as it was before the reaction. ...
... Every chemical reaction involves the rearrangement of atoms into different combinations. However, during these reactions, the total number of atoms of each type of element is the same after the reaction as it was before the reaction. ...
- University at Albany
... Racemization - not always exactly 50/50. Carbocation can be attacked from the top or bottom face giving both enantiomers. Steric hindrance gives attack at one side preferentially Longer-lived carbocations give more racemization, shorter-lived give more inversion ...
... Racemization - not always exactly 50/50. Carbocation can be attacked from the top or bottom face giving both enantiomers. Steric hindrance gives attack at one side preferentially Longer-lived carbocations give more racemization, shorter-lived give more inversion ...
Document
... • Jones Reagent Harsher Oxidant (1° Alcohol Carboxylic Acid) • Alcohol Often Dissolved in Acetone While Jones Reagent Added • Choose Oxidant Based on Desired Carbonyl Functional Group ...
... • Jones Reagent Harsher Oxidant (1° Alcohol Carboxylic Acid) • Alcohol Often Dissolved in Acetone While Jones Reagent Added • Choose Oxidant Based on Desired Carbonyl Functional Group ...
Chemistry 21A: Survey of General and Organic Chemistry
... By the end of the course you should be able to: 1. use the language of general chemistry (vocabulary, nomenclature, formulas and equations) to describe chemical systems and changes (physical and chemical) they undergo. 2. describe the structure of the atom in terms of the arrangement of subatomic pa ...
... By the end of the course you should be able to: 1. use the language of general chemistry (vocabulary, nomenclature, formulas and equations) to describe chemical systems and changes (physical and chemical) they undergo. 2. describe the structure of the atom in terms of the arrangement of subatomic pa ...
Ion exchange chromatography
... to medium salt concentration. The adsorption of the molecules to the solid support is driven by the ionic interaction between the oppositely charged ionic groups in the sample molecule and in the functional ligand on the support. The strength of the interaction is determined by the number and locati ...
... to medium salt concentration. The adsorption of the molecules to the solid support is driven by the ionic interaction between the oppositely charged ionic groups in the sample molecule and in the functional ligand on the support. The strength of the interaction is determined by the number and locati ...
File - wilson science WEBSITE
... If a system at equilibrium is disturbed by adding more NO to the system, which of the following will occur? a. the equilibrium [Cl2] will decrease and K will decrease b. the equilibrium [Cl2] will decrease, and the K will remain the same c. the equilibrium [Cl2] will increase, and the K will increas ...
... If a system at equilibrium is disturbed by adding more NO to the system, which of the following will occur? a. the equilibrium [Cl2] will decrease and K will decrease b. the equilibrium [Cl2] will decrease, and the K will remain the same c. the equilibrium [Cl2] will increase, and the K will increas ...
2011-2012 ACAD REVIEW SHEET Chapter 16
... 24. Describe Le Chatelier’s principle. (ANS: The reaction will shift to minimize the disturbance to reestablish equilibrium.) 25. What factors alter the equilibrium position in chemical reactions? (ANS: Concentration, Pressure and temperature) ...
... 24. Describe Le Chatelier’s principle. (ANS: The reaction will shift to minimize the disturbance to reestablish equilibrium.) 25. What factors alter the equilibrium position in chemical reactions? (ANS: Concentration, Pressure and temperature) ...
Unit 1: Sig. Figs, Compounds, Elements, Homo/Hetero mixtures
... The above orbital notation is used to represent which element? a. Phosphorus b. Arsenic c. Nitrogen d. Silicon 6. Which of the following is the correct configuration notation for the element titanium (Ti)? a. 1s22s22p63s23p64s23d2 b. 1s22s22p63s23p63d24s2 c. 1s22s22p63s23p64s24d2 d. 1s22s22p63s23p64 ...
... The above orbital notation is used to represent which element? a. Phosphorus b. Arsenic c. Nitrogen d. Silicon 6. Which of the following is the correct configuration notation for the element titanium (Ti)? a. 1s22s22p63s23p64s23d2 b. 1s22s22p63s23p63d24s2 c. 1s22s22p63s23p64s24d2 d. 1s22s22p63s23p64 ...
Central Michigan University College of Arts and Sciences Course
... 9. Equilibrium: nature of chemical equilibrium; thermodynamic description; equilibrium calculations for gas phase reactions; magnitude of K and direction of change; Le Chatelier's principle (One week) 10. Acids and bases: classification of acids and bases; Bronsted-Lowry scheme; acid and base streng ...
... 9. Equilibrium: nature of chemical equilibrium; thermodynamic description; equilibrium calculations for gas phase reactions; magnitude of K and direction of change; Le Chatelier's principle (One week) 10. Acids and bases: classification of acids and bases; Bronsted-Lowry scheme; acid and base streng ...
Slide 1
... 1. When two or more substituents are on the same carbon, use the number twice. 2. When two or more substituents are identical, use prefixes 3. When two chains are the same length, use the one with the most substituents on it. 4. If substituents are at equall distances from the ends of the chain, go ...
... 1. When two or more substituents are on the same carbon, use the number twice. 2. When two or more substituents are identical, use prefixes 3. When two chains are the same length, use the one with the most substituents on it. 4. If substituents are at equall distances from the ends of the chain, go ...
Nugget
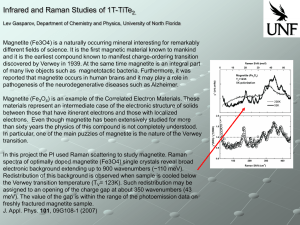
... reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite (Fe3O4) is an example of the Correlated Electron Materials. These materials represent an intermediate case of the electronic structure of solids betwee ...
... reported that magnetite occurs in human brains and it may play a role in pathogenesis of the neurodegenerative diseases such as Alzheimer. Magnetite (Fe3O4) is an example of the Correlated Electron Materials. These materials represent an intermediate case of the electronic structure of solids betwee ...
Chapter 11 Lecture Notes: Alcohols, Ethers, Aldehydes, and Ketones
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...
... group and an -OH group that are bonded to the same carbon. Carbons that are bonded to both an -OR group and an -OH group are called hemiacetal carbons. Carbon number 1 in the ring structure shown meets this criterion. The OH that is bonded to carbon number 1 is obvious, but the OR may not be immedia ...