SCI2199 - Introduction to Organic Chemistry II
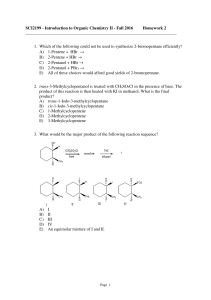
... through use of which of these reagents? A) Concd. HCl B) SO2Cl2 C) NaCl, H2SO4 D) PCl3 E) POCl3 29. Which of the following could be used to synthesize 2-iodobutane? A) CH3CH2CH=CH2 + I2(aq) → B) CH3CH2CHOHCH3 + HI → C) CH3CH2C≡CH + HI → D) CH3CH2C≡CH + I2 → E) None of these choices. ...
... through use of which of these reagents? A) Concd. HCl B) SO2Cl2 C) NaCl, H2SO4 D) PCl3 E) POCl3 29. Which of the following could be used to synthesize 2-iodobutane? A) CH3CH2CH=CH2 + I2(aq) → B) CH3CH2CHOHCH3 + HI → C) CH3CH2C≡CH + HI → D) CH3CH2C≡CH + I2 → E) None of these choices. ...
Synthesis and crystal structure of
... The molecular structure of 1 was determined by X-ray diffraction. The compound is isostructural with the analogous thallium complex. Two monomeric species (Fig. 1, showing the atom numbering scheme) are associated to a “quasi-dimer” via an inversion center between the two In atoms. Three of the five ...
... The molecular structure of 1 was determined by X-ray diffraction. The compound is isostructural with the analogous thallium complex. Two monomeric species (Fig. 1, showing the atom numbering scheme) are associated to a “quasi-dimer” via an inversion center between the two In atoms. Three of the five ...
syntheses, structures, and their interconversion
... PPh4Cl), or Brønstedt bases (AOH, A2CO3) might serve as auxiliary reactants for enhanced hydrogen bonding, and/or to change the basicity. However, only addition of water, or (in reactions of thallium compounds) addition of Brønstedt bases led to the formation of crystalline products (see below). Upo ...
... PPh4Cl), or Brønstedt bases (AOH, A2CO3) might serve as auxiliary reactants for enhanced hydrogen bonding, and/or to change the basicity. However, only addition of water, or (in reactions of thallium compounds) addition of Brønstedt bases led to the formation of crystalline products (see below). Upo ...
Chemistry - Textbooks Online
... The questions that are given at the end of every chapter can be taken only as model questions. A lot of self evaluation questions, like, choose the best answer, one or two sentence answer type and short answer types questions are given in all chapters. While preparing the examination, students shoul ...
... The questions that are given at the end of every chapter can be taken only as model questions. A lot of self evaluation questions, like, choose the best answer, one or two sentence answer type and short answer types questions are given in all chapters. While preparing the examination, students shoul ...
9701/04 - StudyGuide.PK
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
chemistry notes on the mole - lessons
... hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both substances. Water for example is stable, and can be stored easily for long periods of time. Hydrogen peroxide on the other hand must be stored in a dark container because ...
... hydrogen peroxide. Even though this difference seems very small, it results in significant differences in the properties of both substances. Water for example is stable, and can be stored easily for long periods of time. Hydrogen peroxide on the other hand must be stored in a dark container because ...
Proceedings of the Indiana Academy of Science
... inclusion of a larger number of functional groups introduces more organic reactions and affords a broader view of the subject. Salts of amines and carboxylic acids are excluded as possibilities in the identification procedures. The chemical principles of the tests are outlined within the experiment; ...
... inclusion of a larger number of functional groups introduces more organic reactions and affords a broader view of the subject. Salts of amines and carboxylic acids are excluded as possibilities in the identification procedures. The chemical principles of the tests are outlined within the experiment; ...
2016 - Specimen Paper 4 - Cambridge International Examinations
... number of moles of HCl used = ………………………………………………...…..……. number of moles of CoCl2 formed = ……………………………………………..….…… number of moles of CoCl2.6H2O formed = ………………………………………..…..… mass of one mole of CoCl2.6H2O = 238 g maximum yield of CoCl2.6H2O = …………………………………………………..…..… g to show that cobalt(II) ca ...
... number of moles of HCl used = ………………………………………………...…..……. number of moles of CoCl2 formed = ……………………………………………..….…… number of moles of CoCl2.6H2O formed = ………………………………………..…..… mass of one mole of CoCl2.6H2O = 238 g maximum yield of CoCl2.6H2O = …………………………………………………..…..… g to show that cobalt(II) ca ...
TOPIC 6. NUCLEOPHILIC SUBSTITUTIONS (chapter 6 and parts of
... molecules in one step by substitution of an appropriate material 4. Explore the substitution chemistry of alcohols and ethers ...
... molecules in one step by substitution of an appropriate material 4. Explore the substitution chemistry of alcohols and ethers ...
EXPERIMENT 2 Properties of Alkanes, Alkenes, and Alcohols
... Introduction A main focus of much of this course is on how the structure of organic molecules determines their properties, both physical and chemical. Physical properties include such things as melting point (mp), boiling point (bp), refractive index (nD), density (d), and optical rotation (αD). Sol ...
... Introduction A main focus of much of this course is on how the structure of organic molecules determines their properties, both physical and chemical. Physical properties include such things as melting point (mp), boiling point (bp), refractive index (nD), density (d), and optical rotation (αD). Sol ...
The Chemical Context of Life
... In radiometric dating, scientists measure the ratio of different isotopes and calculate how many half-lives have passed since the fossil or rock was ...
... In radiometric dating, scientists measure the ratio of different isotopes and calculate how many half-lives have passed since the fossil or rock was ...
High Oxygen Pressures and the Stabilization of the Highest
... the first row transition metals stabilized under oxygen pressures in specific structures derived from the perovskite type. The stabilization of such oxidation states depends not only on the applied oxygen pressure but also on several chemical and structural parameters. Transition metal oxides with h ...
... the first row transition metals stabilized under oxygen pressures in specific structures derived from the perovskite type. The stabilization of such oxidation states depends not only on the applied oxygen pressure but also on several chemical and structural parameters. Transition metal oxides with h ...
Calculations with Chemical Formulas and Equations
... analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
... analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined ...
AP Chemistry
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...
... 2. surroundings lose energy (cool down) b. when energy required to break bonds < energy released to form new bonds, –H (exothermic) 1. products at a lower energy state than reactants (stronger bonds) 2. surroundings gain energy (heat up) 5. thermochemical equation a. chemical equation with H 1. li ...























