sch103manual - university of nairobi staff profiles
... any of the three states of matter: Solids, liquid or gas. Water for example, exists in the solid state as ice, liquid state as water and in the gaseous state as steam. The physical properties of a substance often depend on the state of the substance. In this section, we will review the states of mat ...
... any of the three states of matter: Solids, liquid or gas. Water for example, exists in the solid state as ice, liquid state as water and in the gaseous state as steam. The physical properties of a substance often depend on the state of the substance. In this section, we will review the states of mat ...
Chem12 Buffer/Titration : Probs
... added to the system., 57) 1.8x10-4 , 58) c, 59) d, 60) c, 61) b, 62) c, 63) Do a titration with NaOH. See how much NaOH solution is required to reach the equivalence point for each acid. Use an indicator(s) to find the pH when the equivalence point is reached. The lower the pH at the equivalence poi ...
... added to the system., 57) 1.8x10-4 , 58) c, 59) d, 60) c, 61) b, 62) c, 63) Do a titration with NaOH. See how much NaOH solution is required to reach the equivalence point for each acid. Use an indicator(s) to find the pH when the equivalence point is reached. The lower the pH at the equivalence poi ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Precipitation problem: When aqueous silver nitrate and sodium chromate solutions are mixed, solid silver chromate forms in a solution of sodium nitrate. If 257.8 mL of a 0.0468 M solution of silver nitrate is added to 156.00 mL of a 0.0950 M solution of sodium chromate, what mass of silver chromate ...
... Precipitation problem: When aqueous silver nitrate and sodium chromate solutions are mixed, solid silver chromate forms in a solution of sodium nitrate. If 257.8 mL of a 0.0468 M solution of silver nitrate is added to 156.00 mL of a 0.0950 M solution of sodium chromate, what mass of silver chromate ...
Final Review 3-8 Answers_2
... Measure 20 mL of MgSO4(aq) (stock solution) using a pipette. Transfer the stock solution slowly into the volumetric flask while mixing. Add pure water, and then use a medicine dropper to set the bottom of the meniscus on the calibration line. ...
... Measure 20 mL of MgSO4(aq) (stock solution) using a pipette. Transfer the stock solution slowly into the volumetric flask while mixing. Add pure water, and then use a medicine dropper to set the bottom of the meniscus on the calibration line. ...
Calculations with Chemical Formulas and Equations
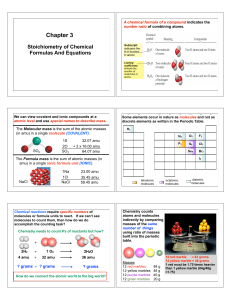
... • The atomic mass unit is defined this way. • How many 12C atoms weigh 12 g? • 6.02x1023 12C weigh 12 g. • Avagodro’s number • The mole Stoichiometry ...
... • The atomic mass unit is defined this way. • How many 12C atoms weigh 12 g? • 6.02x1023 12C weigh 12 g. • Avagodro’s number • The mole Stoichiometry ...
File
... calculated values. If graphs are included, make the graphs an appropriate size. Label all axes and give each graph a title. If experiments are not quantitative, this section may be omitted. 8. Conclusions – Make a simple statement concerning what you conclude from the experiment. This is not a place ...
... calculated values. If graphs are included, make the graphs an appropriate size. Label all axes and give each graph a title. If experiments are not quantitative, this section may be omitted. 8. Conclusions – Make a simple statement concerning what you conclude from the experiment. This is not a place ...
AP Chemistry Lab Manual
... calculated values. If graphs are included, make the graphs an appropriate size. Label all axes and give each graph a title. If experiments are not quantitative, this section may be omitted. 8. Conclusions – Make a simple statement concerning what you conclude from the experiment. This is not a place ...
... calculated values. If graphs are included, make the graphs an appropriate size. Label all axes and give each graph a title. If experiments are not quantitative, this section may be omitted. 8. Conclusions – Make a simple statement concerning what you conclude from the experiment. This is not a place ...
CHAPTER 4 - Myschoolpages.com
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
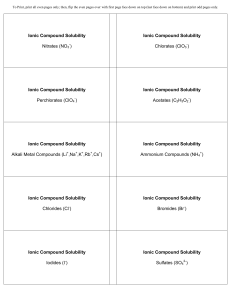
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
... Definition A substance that is able to donate a H+ ion (a proton) and, hence, increases the concentration of H+(aq) when it dissolves in water. ...
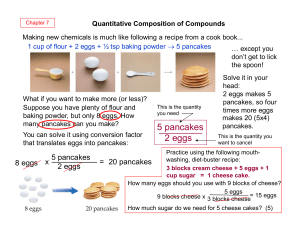
5 pancakes 2 eggs
... Example 12: Calculate the # molecules of NH3 formed by the reaction of 150. g H2. 1 mole H2 2 moles NH3 6.022 x 1023 molecules NH3 = 2.99 x 1025 150. g H2 x x x 2.016 g H2 3 moles H2 1 mole NH3 molecules NH3. Starting Step 1 Step 3 result compound Step 2 Limiting Reactant and Yield Calculations The ...
... Example 12: Calculate the # molecules of NH3 formed by the reaction of 150. g H2. 1 mole H2 2 moles NH3 6.022 x 1023 molecules NH3 = 2.99 x 1025 150. g H2 x x x 2.016 g H2 3 moles H2 1 mole NH3 molecules NH3. Starting Step 1 Step 3 result compound Step 2 Limiting Reactant and Yield Calculations The ...
class notes 4
... Acid-Base Reactions (Acid-Base Reactions Always Go) Acid: Substance that produces H+ ions in aqueous solution is the Arrhenius definition of acid. Base: Substance that produces OH- in aqueous solution is the Arrhenius definition of base. Actually a hydrogen ion is a bare proton and will associate wi ...
... Acid-Base Reactions (Acid-Base Reactions Always Go) Acid: Substance that produces H+ ions in aqueous solution is the Arrhenius definition of acid. Base: Substance that produces OH- in aqueous solution is the Arrhenius definition of base. Actually a hydrogen ion is a bare proton and will associate wi ...
Chapter 15 - Cengage Learning
... 15. In this problem we are asked to calculate how much of a concentrated stock solution, which is 12 M HCl, is needed to prepare a dilute HCl solution. We will need to know how many moles of HCl are present in 2.5 L of 1.0 M HCl, that is, in the dilute solution. Then we need to find a volume of the ...
... 15. In this problem we are asked to calculate how much of a concentrated stock solution, which is 12 M HCl, is needed to prepare a dilute HCl solution. We will need to know how many moles of HCl are present in 2.5 L of 1.0 M HCl, that is, in the dilute solution. Then we need to find a volume of the ...