Chapter 4 Chemical Quantities and Aqueous Reactions
... • other ionic compounds, like AgCl, dissolve hardly at all in water at room temperature • compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble – NaCl is soluble in water, AgCl is insoluble in water – the degree of solubility depends on the tem ...
... • other ionic compounds, like AgCl, dissolve hardly at all in water at room temperature • compounds that dissolve in a solvent are said to be soluble, while those that do not are said to be insoluble – NaCl is soluble in water, AgCl is insoluble in water – the degree of solubility depends on the tem ...
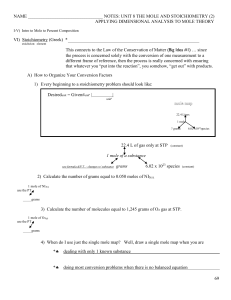
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... Meaning: For every 2 mol of ethane, 7 mol of O2 molecules are consumed in the combustion THUS, producing 4 mol of carbon dioxide and 6 moles of water with the release of 3,170 kJ of thermal energy into the environment. This set of relationships can indicate, at a glance that: Twice as many moles of ...
... Meaning: For every 2 mol of ethane, 7 mol of O2 molecules are consumed in the combustion THUS, producing 4 mol of carbon dioxide and 6 moles of water with the release of 3,170 kJ of thermal energy into the environment. This set of relationships can indicate, at a glance that: Twice as many moles of ...
Preparatory Problems of the 40th IChO - IChO-2016
... Relation between equilibrium constants, electromotive force and standard Gibbs energy; ...
... Relation between equilibrium constants, electromotive force and standard Gibbs energy; ...
Solving Systems of Linear Equations
... Solve for x in one of the equations. Substitute the expression for x into the other equation to find y. Then substitute the value of y into one of the original equations to find x. Method 2 Solve for y first. Solve for y in one of the equations. Substitute the expression for y into the other equatio ...
... Solve for x in one of the equations. Substitute the expression for x into the other equation to find y. Then substitute the value of y into one of the original equations to find x. Method 2 Solve for y first. Solve for y in one of the equations. Substitute the expression for y into the other equatio ...
Calculations and the Chemical Equation
... Just as a mole of atoms is based on the atomic mass or atomic weight, a mole of a compound is based upon the formula mass or formula weight. To calculate the formula weight, the formula unit must be known. The Chemical Equation and the Information it Conveys In a chemical equation, the identity of r ...
... Just as a mole of atoms is based on the atomic mass or atomic weight, a mole of a compound is based upon the formula mass or formula weight. To calculate the formula weight, the formula unit must be known. The Chemical Equation and the Information it Conveys In a chemical equation, the identity of r ...
Solving Systems of Linear Equations
... Solve for x in one of the equations. Substitute the expression for x into the other equation to find y. Then substitute the value of y into one of the original equations to find x. Method 2 Solve for y first. Solve for y in one of the equations. Substitute the expression for y into the other equatio ...
... Solve for x in one of the equations. Substitute the expression for x into the other equation to find y. Then substitute the value of y into one of the original equations to find x. Method 2 Solve for y first. Solve for y in one of the equations. Substitute the expression for y into the other equatio ...
Stoichiometry Worksheet
... 3. Convert all amounts of products and/or reactants in the question into moles. Balance the following equation and use it to work the problems! NH3 + O2 → NO + H2O 4NH3 + 5O2 → 4NO + 6H2O a. How many grams of NO were needed to produce 30.2 g of water? ...
... 3. Convert all amounts of products and/or reactants in the question into moles. Balance the following equation and use it to work the problems! NH3 + O2 → NO + H2O 4NH3 + 5O2 → 4NO + 6H2O a. How many grams of NO were needed to produce 30.2 g of water? ...
Unit 4 - Chemical Equilibrium
... A _ _ _ _ _ _ _ _ _ called Le Chatelier _ _ _ _ _ _ _ _ _ _ _ _ what happens when the conditions of reactions in _ _ _ _ _ _ _ _ _ _ _ are changed. He developed this _ _ _ _ _ _ _ _ _: 'If a reaction in equilibrium is subjected to a change, then it responds in such a way as to _ _ _ _ _ _ _ _ _ _ th ...
... A _ _ _ _ _ _ _ _ _ called Le Chatelier _ _ _ _ _ _ _ _ _ _ _ _ what happens when the conditions of reactions in _ _ _ _ _ _ _ _ _ _ _ are changed. He developed this _ _ _ _ _ _ _ _ _: 'If a reaction in equilibrium is subjected to a change, then it responds in such a way as to _ _ _ _ _ _ _ _ _ _ th ...
study guide spring 2012
... d. number of products. A chemical equation is balanced when the a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants ...
... d. number of products. A chemical equation is balanced when the a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants ...
- Academy Test Bank
... -(CH2CHCN)n-, where n is typically greater than 10,000. Given that a sample of monodisperse polyacrilonitrile weighs 197.4 g and contains 1.046 10 20 molecules of -(CH2CHCN)n-, calculate n. A) 2.141 10 4 B) 6.026 10 7 C) 6.018 10 39 D) 6.022 10 23 E) 1.136 10 6 ANS: A PTS: 1 DIF: difficu ...
... -(CH2CHCN)n-, where n is typically greater than 10,000. Given that a sample of monodisperse polyacrilonitrile weighs 197.4 g and contains 1.046 10 20 molecules of -(CH2CHCN)n-, calculate n. A) 2.141 10 4 B) 6.026 10 7 C) 6.018 10 39 D) 6.022 10 23 E) 1.136 10 6 ANS: A PTS: 1 DIF: difficu ...