Lecture 1 and 2 Volumetric analysis Zuhair Khammas
... These can be subdivided into the following principal methods; 1- Neutralization: The process in which a solution of one reactant, the titrant, is carefully added to a solution of another reactant, and the volume of titrant required for complete reaction is measured. i.e. these are based on reaction ...
... These can be subdivided into the following principal methods; 1- Neutralization: The process in which a solution of one reactant, the titrant, is carefully added to a solution of another reactant, and the volume of titrant required for complete reaction is measured. i.e. these are based on reaction ...
Vinnitsa National Pirogov Memorial Medical University Biological
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
Supplementary Exercise 1B Topic 5
... In the electrochemical series, the position of calcium is higher than that of sodium. The order is different from that in the reactivity series. This is because calcium atom loses electrons more readily in cell reactions than in reaction with air, water and dilute acids. ...
... In the electrochemical series, the position of calcium is higher than that of sodium. The order is different from that in the reactivity series. This is because calcium atom loses electrons more readily in cell reactions than in reaction with air, water and dilute acids. ...
odd - WWW2
... The three classes are ionic, covalent, and metallic. Ionic carbides are formed by the most electropositive metals. These may contain the dicarbide(2 ) ion, C22 , or the true carbide ion C4 . Both types of ionic carbides react with water to produce the appropriate hydrocarbon. Covalent carbides are f ...
... The three classes are ionic, covalent, and metallic. Ionic carbides are formed by the most electropositive metals. These may contain the dicarbide(2 ) ion, C22 , or the true carbide ion C4 . Both types of ionic carbides react with water to produce the appropriate hydrocarbon. Covalent carbides are f ...
1 Acids and Bases
... Many chemists still use H+ (aq) to represent this situation, but it is understood that this is just an abbreviation for what is really occurring in solution. You are likely to come across both, and it is important for you to understand that they are actually describing the same entity. When using th ...
... Many chemists still use H+ (aq) to represent this situation, but it is understood that this is just an abbreviation for what is really occurring in solution. You are likely to come across both, and it is important for you to understand that they are actually describing the same entity. When using th ...
U3 Student Workbook - The Connected Chemistry Curriculum
... This lesson contains four activities that introduce students to five different kinds of chemical reactions (combination, decomposition, single displacement, double displacement, and combustion reactions). In the Connecting Activity, students apply the Law of Conservation of Mass to chemical equation ...
... This lesson contains four activities that introduce students to five different kinds of chemical reactions (combination, decomposition, single displacement, double displacement, and combustion reactions). In the Connecting Activity, students apply the Law of Conservation of Mass to chemical equation ...
Solving Linear Congruences
... Let me summarize what I’ve just shown. I’ve proven that two solutions of the above form are equal mod m if and only if their parameter values are equal mod d. That is, if I let t range over a complete system m of residues mod d, then x0 + t ranges over all possible solutions mod m. To be very speci ...
... Let me summarize what I’ve just shown. I’ve proven that two solutions of the above form are equal mod m if and only if their parameter values are equal mod d. That is, if I let t range over a complete system m of residues mod d, then x0 + t ranges over all possible solutions mod m. To be very speci ...
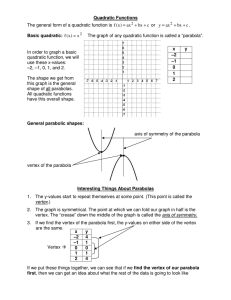
A. y - cloudfront.net
... A video club charges $8 to join, and $1.25 for each DVD that is rented. The linear equation y = 1.25x + 8 represents the amount of money y spent after renting x DVDs. Graph the equation by first identifying the slope and y-intercept. y = 1.25x + 8 m =1.25 ...
... A video club charges $8 to join, and $1.25 for each DVD that is rented. The linear equation y = 1.25x + 8 represents the amount of money y spent after renting x DVDs. Graph the equation by first identifying the slope and y-intercept. y = 1.25x + 8 m =1.25 ...
Chapter 1: Chemistry: The Study of Change
... 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of ...
... 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of ...