Overview of Metabolism Chapter
... production of ATP to occur in the absence of oxygen. By oxidizing the NADH produced in glycolysis, fermentation regenerates NAD+, which can take part in glycolysis once again to produce more ATP. The muscle regenerates NAD+ from NADH, an oxidation reaction, by reduction of pyruvate. The fermentation ...
... production of ATP to occur in the absence of oxygen. By oxidizing the NADH produced in glycolysis, fermentation regenerates NAD+, which can take part in glycolysis once again to produce more ATP. The muscle regenerates NAD+ from NADH, an oxidation reaction, by reduction of pyruvate. The fermentation ...
Consortium for Educational Communication
... form of a reduced pyridine nucleotide, NADH. Both glycolysis and TCA cycle takes place in the inner membrane of mitochondrion. Tricarboxylic acid cycle: When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, two processes can oc ...
... form of a reduced pyridine nucleotide, NADH. Both glycolysis and TCA cycle takes place in the inner membrane of mitochondrion. Tricarboxylic acid cycle: When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, two processes can oc ...
Luminescent Calcium Instructions
... generating a green light flash. It is primarily used in reporter assays and ATP assays. Its bioluminescent reaction is the most efficient known in nature! (With about 90 % of the energy released being converted to light): ATPdependent oxidation of luciferin by luciferase results in bioluminescence ( ...
... generating a green light flash. It is primarily used in reporter assays and ATP assays. Its bioluminescent reaction is the most efficient known in nature! (With about 90 % of the energy released being converted to light): ATPdependent oxidation of luciferin by luciferase results in bioluminescence ( ...
Aminosav metabolizmus IV. Aminosavak bioszintézise
... Porphyrias: group of genetic diseases caused by the accumulation in body fluids, and liver of some porphyrin precursors (because of the defect of certain enzyme in the biosynthesis of porphyrin). ...
... Porphyrias: group of genetic diseases caused by the accumulation in body fluids, and liver of some porphyrin precursors (because of the defect of certain enzyme in the biosynthesis of porphyrin). ...
photosynthesis workbook lesson
... through electron transport chains, a series of electron carrier proteins. • The movement of electrons through an electron transport chain causes a thylakoid to fill up with hydrogen ions and generates ATP and NADPH. • ATP synthase is a membrane protein through which excess hydrogen ions escape a thy ...
... through electron transport chains, a series of electron carrier proteins. • The movement of electrons through an electron transport chain causes a thylakoid to fill up with hydrogen ions and generates ATP and NADPH. • ATP synthase is a membrane protein through which excess hydrogen ions escape a thy ...
BOOK NOTES ch9_sec3
... Efficiency of Cellular Respiration • Cells release energy most efficiently when oxygen is present because they make most of their ATP during aerobic respiration. ...
... Efficiency of Cellular Respiration • Cells release energy most efficiently when oxygen is present because they make most of their ATP during aerobic respiration. ...
The Working Cell
... therefore oxidized. • 3. At the end of cellular respiration, glucose has been oxidized to carbon dioxide and water and ATP molecules have been produced. • In metabolic pathways, most oxidations involve the coenzyme NAD+ (nicotinamide adenine dinucleotide); the molecule accepts two electrons but only ...
... therefore oxidized. • 3. At the end of cellular respiration, glucose has been oxidized to carbon dioxide and water and ATP molecules have been produced. • In metabolic pathways, most oxidations involve the coenzyme NAD+ (nicotinamide adenine dinucleotide); the molecule accepts two electrons but only ...
2. The citric acid cycle
... NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
... NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
Ch 9 Text Study Guide
... 29. Why does a sprinter have an oxygen debt to repay after the race is over? ...
... 29. Why does a sprinter have an oxygen debt to repay after the race is over? ...
Respiration and Metabolism
... NAD NADH (Reduced/Oxidized) Carried to ETC : at Glycolysis, Krebs cycle NADH NAD (Reduced/Oxidized) : at ETC ( Electron Transport Chain ) *The more reduced = the more energy it holds. Reduced coenzymes carry high-energy electrons to proton pumps where ATP is then made( ETC). ...
... NAD NADH (Reduced/Oxidized) Carried to ETC : at Glycolysis, Krebs cycle NADH NAD (Reduced/Oxidized) : at ETC ( Electron Transport Chain ) *The more reduced = the more energy it holds. Reduced coenzymes carry high-energy electrons to proton pumps where ATP is then made( ETC). ...
Pyruvate Oxidation and the Citric Acid Cycle
... Succinate is oxidized to fumarate, with the formation of FADH2. Succinyl CoA releases coenzyme A, becoming succinate, the energy thus released converts GDP to GTP, which in turn converts ADP to ATP. ...
... Succinate is oxidized to fumarate, with the formation of FADH2. Succinyl CoA releases coenzyme A, becoming succinate, the energy thus released converts GDP to GTP, which in turn converts ADP to ATP. ...
bioc-2200-a-biol-2200-a-mock-final-exam
... c. allow for the reoxidation of NADH d. allow the complete oxidation of glucose 7. Which of the following reactions of the Citric Acid Cycle produces CO2? a. the formation of α-ketoglutarate b. the formation of succinate c. the formation of isocitrate d. the formation of oxaloacetate 8. In a newly d ...
... c. allow for the reoxidation of NADH d. allow the complete oxidation of glucose 7. Which of the following reactions of the Citric Acid Cycle produces CO2? a. the formation of α-ketoglutarate b. the formation of succinate c. the formation of isocitrate d. the formation of oxaloacetate 8. In a newly d ...
Metabolism
... 4. Describe how enzymes act as catalysts of reactions with reference to the reaction catalysed by lysozyme. In a biological setting, most energetically favourable reactions will not occur at a rate useful for life, unless catalysed by enzymes. Enzymes function by lowering the barriers that block a p ...
... 4. Describe how enzymes act as catalysts of reactions with reference to the reaction catalysed by lysozyme. In a biological setting, most energetically favourable reactions will not occur at a rate useful for life, unless catalysed by enzymes. Enzymes function by lowering the barriers that block a p ...
Plant Respiration Exchange of Gases in Plants - E
... Electrons from NADH (produced in the mitochondria matrix) are oxidized by an NADH dehydrogenase (Complex I). After that, electrons are transferred to ubiquinone which is located within the inner membrane. Ubiquinone also receives reducing equivalents via FADH2 (Complex II). FADH2 is generated during ...
... Electrons from NADH (produced in the mitochondria matrix) are oxidized by an NADH dehydrogenase (Complex I). After that, electrons are transferred to ubiquinone which is located within the inner membrane. Ubiquinone also receives reducing equivalents via FADH2 (Complex II). FADH2 is generated during ...
BioN08 Metabolism of lipids Summer 2015
... • The purpose is to produce coenzymes (NADH and FADH2) that will enter the elelctron transport chain and produce ATP. • This is done in four stages: – Activation – Transport – Beta-oxidation ...
... • The purpose is to produce coenzymes (NADH and FADH2) that will enter the elelctron transport chain and produce ATP. • This is done in four stages: – Activation – Transport – Beta-oxidation ...
BDS Ist YEAR EXAMINATION 2008-09
... Cyanide is toxic because it: a) Inhibits cytochrome C oxidase b) Forms cyanmethaemoglobin c) Inhibits ATP carrier in mitochondria d) Inhibits Na+-K+ ATPase ...
... Cyanide is toxic because it: a) Inhibits cytochrome C oxidase b) Forms cyanmethaemoglobin c) Inhibits ATP carrier in mitochondria d) Inhibits Na+-K+ ATPase ...
Document
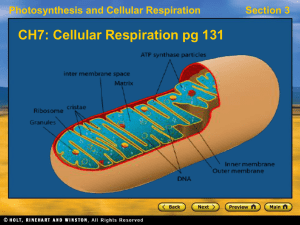
... area of the inner mitochondrial membrane, enhancing its ability to generate ATP. The matrix is the space enclosed by the inner membrane. The matrix contains a highly concentrated mixture of hundreds of enzymes, which the major functions include oxidation of pyruvate and fatty acids, and the citric a ...
... area of the inner mitochondrial membrane, enhancing its ability to generate ATP. The matrix is the space enclosed by the inner membrane. The matrix contains a highly concentrated mixture of hundreds of enzymes, which the major functions include oxidation of pyruvate and fatty acids, and the citric a ...
Cellular Respiration
... – 2 molecules NADH are created • Important because NADH are Hydrogen ion/proton and e- carriers ...
... – 2 molecules NADH are created • Important because NADH are Hydrogen ion/proton and e- carriers ...
Energy, ATP, and Enzymes Energy - the ability to do work, that is, to
... An enzyme can distinguish its substrate from similar molecules and even isomers of the same molecule Only a restricted region of the enzyme molecule actually binds to the substrate this is called the active site o This match is not perfect - as the enzyme and substrate come together, a small conform ...
... An enzyme can distinguish its substrate from similar molecules and even isomers of the same molecule Only a restricted region of the enzyme molecule actually binds to the substrate this is called the active site o This match is not perfect - as the enzyme and substrate come together, a small conform ...
Chapter 3
... • In cells, often involve the transfer of hydrogen atoms rather than free electrons – Hydrogen atom contains one electron – A molecule that loses a hydrogen also loses an electron and, therefore, is oxidized © 2007 McGraw-Hill Higher Education. All rights reserved. ...
... • In cells, often involve the transfer of hydrogen atoms rather than free electrons – Hydrogen atom contains one electron – A molecule that loses a hydrogen also loses an electron and, therefore, is oxidized © 2007 McGraw-Hill Higher Education. All rights reserved. ...
Cellular Respiration and Photosynthesis
... *Glycolysis takes place in the cytoplasm. During glycolysis, 1 glucose molecule is broken down into 2 molecules of pyruvic acid. *4 ATP molecules are formed; however, glycolysis requires 2 ATP to break apart each molecule of glucose; therefore, the net energy produced during glycolysis is 2 ATP. * I ...
... *Glycolysis takes place in the cytoplasm. During glycolysis, 1 glucose molecule is broken down into 2 molecules of pyruvic acid. *4 ATP molecules are formed; however, glycolysis requires 2 ATP to break apart each molecule of glucose; therefore, the net energy produced during glycolysis is 2 ATP. * I ...
03-232 Biochemistry ... Name:________________________ or the back of the preceding page. In questions... Instructions:
... Choice B: Cholesterol keeps the membrane fluid, permitting conformational changes to occur in membrane enzymes and the diffusion of electron carriers, such as CoQ. Choice C: Both convert substrate to product. A soluble enzyme will typically cause a change in the chemical structure of the substrate. ...
... Choice B: Cholesterol keeps the membrane fluid, permitting conformational changes to occur in membrane enzymes and the diffusion of electron carriers, such as CoQ. Choice C: Both convert substrate to product. A soluble enzyme will typically cause a change in the chemical structure of the substrate. ...
CHAPTERS 6 & 7
... – As a result, ATP is generated through oxidative phosphorylation (OXYGEN is REQUIRED = AEROBIC) – This stage occurs in the mitochondria – Another 36 ATPs are produced in stages 2 and 3 ...
... – As a result, ATP is generated through oxidative phosphorylation (OXYGEN is REQUIRED = AEROBIC) – This stage occurs in the mitochondria – Another 36 ATPs are produced in stages 2 and 3 ...
03. Metabolism of lipids
... Fatty Acids • TGs are delivered to adipose tissue in the form of chylomicrones and VLDL, hydrolyzed by lipoprotein lipase into fatty acids and glycerol, which are taken up by adipocytes. • Then fatty acids are reesterified to TGs. • TGs are stored in adipocytes. • To supply energy demands fatty acid ...
... Fatty Acids • TGs are delivered to adipose tissue in the form of chylomicrones and VLDL, hydrolyzed by lipoprotein lipase into fatty acids and glycerol, which are taken up by adipocytes. • Then fatty acids are reesterified to TGs. • TGs are stored in adipocytes. • To supply energy demands fatty acid ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.