- PlanbookConnect
... in the form of CO2. The Hydrogens are being carried by NADH and FADH2 to the ETC. ...
... in the form of CO2. The Hydrogens are being carried by NADH and FADH2 to the ETC. ...
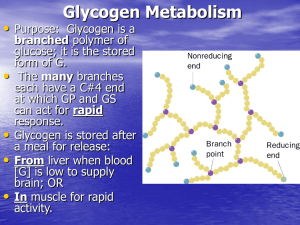
Glycogen Metabolism - http://www.utm.edu
... 2. Adrenalin (epinephrine) is a signal to “break down” muscle glycogen to produce G6P for ATP production (in G’lys and in OP via PDH, TCA, ET and OP) for fightor-flight. Muscle GP is activated, GS inhibited. When either one binds its cell-membrane receptor: a. the hormone-receptor complex binds to a ...
... 2. Adrenalin (epinephrine) is a signal to “break down” muscle glycogen to produce G6P for ATP production (in G’lys and in OP via PDH, TCA, ET and OP) for fightor-flight. Muscle GP is activated, GS inhibited. When either one binds its cell-membrane receptor: a. the hormone-receptor complex binds to a ...
Enzymes
... Adding energy to a substance makes it more reactive For different reactions different energy thresholds are needed Enzymes lower that threshold ...
... Adding energy to a substance makes it more reactive For different reactions different energy thresholds are needed Enzymes lower that threshold ...
Electron Transport Chain
... In aerobic cellular respiration cells take in sugar (glucose) and breaks it down to into carbon dioxide and water, this requires oxygen. This process produces energy in the form of ATP C6H12O6 + 6O2 → 6CO2 +6H2O + Energy ...
... In aerobic cellular respiration cells take in sugar (glucose) and breaks it down to into carbon dioxide and water, this requires oxygen. This process produces energy in the form of ATP C6H12O6 + 6O2 → 6CO2 +6H2O + Energy ...
Photosynthesis
... make 3 carbon sugars from CO2 Used to make more complex sugars or other biochemical molecules ...
... make 3 carbon sugars from CO2 Used to make more complex sugars or other biochemical molecules ...
Chemistry 326 Name_____________________ Fall 2009 Check
... d. two moles of NADH and four moles of ATP e. two moles of NAD+ and four moles of ATP 6. The steps of glycolysis between glyceraldehyde-3-phosphate and 3phosphoglycerate involve all of the following except: a. ATP synthesis d. the formation of 1,3bisphosphoglycerate b. utilization of Pi e. catalysis ...
... d. two moles of NADH and four moles of ATP e. two moles of NAD+ and four moles of ATP 6. The steps of glycolysis between glyceraldehyde-3-phosphate and 3phosphoglycerate involve all of the following except: a. ATP synthesis d. the formation of 1,3bisphosphoglycerate b. utilization of Pi e. catalysis ...
Enzymes & Photosynthesis
... • The quantities are mind boggling. A hectare (e.g. a field 100 m by 100 m) of wheat can convert as much as 10,000 kg of carbon from carbon dioxide into the carbon of sugar in a year, giving a total yield of 25,000 kg of sugar per year. • There is a total of 7000 x 109 tonnes of carbon dioxide in t ...
... • The quantities are mind boggling. A hectare (e.g. a field 100 m by 100 m) of wheat can convert as much as 10,000 kg of carbon from carbon dioxide into the carbon of sugar in a year, giving a total yield of 25,000 kg of sugar per year. • There is a total of 7000 x 109 tonnes of carbon dioxide in t ...
Lecture 7 Citric acid cycle
... Whether glucose 6-phosphate enters glycolysis or the phosphate pathway depends on the current needs of the cell and on the concentration of NADP in cytosol. ...
... Whether glucose 6-phosphate enters glycolysis or the phosphate pathway depends on the current needs of the cell and on the concentration of NADP in cytosol. ...
09_Lecture_Presentation
... In that case, glycolysis couples with anaerobic respiration or fermentation to produce ATP ...
... In that case, glycolysis couples with anaerobic respiration or fermentation to produce ATP ...
2-Phospho
... phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via ...
... phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via ...
Cellular Metabolism
... A. ___________ refers to all of the chemical reactions in an organism. Two types of metabolism are 1. ___________ – the break down of large molecules to smaller ones. Catabolic reactions are ___________ (they release energy) 2. __________ – the build up of large molecules from smaller ones. Anabolic ...
... A. ___________ refers to all of the chemical reactions in an organism. Two types of metabolism are 1. ___________ – the break down of large molecules to smaller ones. Catabolic reactions are ___________ (they release energy) 2. __________ – the build up of large molecules from smaller ones. Anabolic ...
Lecture 3
... NADH: Role during slow glycolysis • NADH (reduced form) must be “shuttled” into the mitochondria (electron transport chain) • NAD+ is recycled during the shuttling process • This provides enough NAD+ to maintain glycolysis at slower rates of energy turnover ...
... NADH: Role during slow glycolysis • NADH (reduced form) must be “shuttled” into the mitochondria (electron transport chain) • NAD+ is recycled during the shuttling process • This provides enough NAD+ to maintain glycolysis at slower rates of energy turnover ...
UNIT-I (VSAQ) Chapter-4 Photosynthesis in Higher Plants
... electron. When this molecule passes on its electron to the electron carrier on the inner side of the membrane, the proto is released into the inner side or the lumen side of the membrane. (c) The NADP reductase enzyme is located on the stroma side of the membrane. Along with electrons that come from ...
... electron. When this molecule passes on its electron to the electron carrier on the inner side of the membrane, the proto is released into the inner side or the lumen side of the membrane. (c) The NADP reductase enzyme is located on the stroma side of the membrane. Along with electrons that come from ...
Chapter 9
... 15. What happens during electron transport? 16. Why do electrons NEED to “break the fall?” 17. How is ATP made during chemiosmosis? 18. What happens when there is no O2? 19. How do the other foods we eat get catabolized? 20. How is cellular respiration controlled? ...
... 15. What happens during electron transport? 16. Why do electrons NEED to “break the fall?” 17. How is ATP made during chemiosmosis? 18. What happens when there is no O2? 19. How do the other foods we eat get catabolized? 20. How is cellular respiration controlled? ...
Cellular Respiration chapt06
... The Krebs Cycle begins with pyruvate (from Glycolysis) Remember, there are 2 pyruvates made from each Glucose, so there are 2 Krebs Cycles for every glucose molecule During a Kreb’s Cycle the pyruvate (a 3 carbon molecule) will be completely oxidized to become 3 carbon dioxide molecules (one carbon ...
... The Krebs Cycle begins with pyruvate (from Glycolysis) Remember, there are 2 pyruvates made from each Glucose, so there are 2 Krebs Cycles for every glucose molecule During a Kreb’s Cycle the pyruvate (a 3 carbon molecule) will be completely oxidized to become 3 carbon dioxide molecules (one carbon ...
Cellular Respiration
... Glycolysis is the process in which one molecule of glucose is broken in half, producing two molecules of pyruvic acid, a 3carbon compound. How much ATP is released during glycolysis? ...
... Glycolysis is the process in which one molecule of glucose is broken in half, producing two molecules of pyruvic acid, a 3carbon compound. How much ATP is released during glycolysis? ...
Chapter 8: An Introduction to Metabolism
... In terms of the energy in a system, the only thing we are concerned with is the free energy--known as the Gibbs Free Energy. Gibbs Free Energy is the energy that is available to do work. ...
... In terms of the energy in a system, the only thing we are concerned with is the free energy--known as the Gibbs Free Energy. Gibbs Free Energy is the energy that is available to do work. ...
Completed notes
... Skeletal muscles produce lactic acid when the body cannot supply enough oxygen, such as during periods of strenuous exercise. ...
... Skeletal muscles produce lactic acid when the body cannot supply enough oxygen, such as during periods of strenuous exercise. ...
213lec3
... A. Metabolic pathways are sequences of reactions that are catalyzed by enzymes. Metabolic pathways allow for the slow and controlled breakdown of foodstuffs so that energy can be captured and used to form ATP. B. Glycolysis: Breaks glucose into two pyruvate molecules. After glycolysis but before ent ...
... A. Metabolic pathways are sequences of reactions that are catalyzed by enzymes. Metabolic pathways allow for the slow and controlled breakdown of foodstuffs so that energy can be captured and used to form ATP. B. Glycolysis: Breaks glucose into two pyruvate molecules. After glycolysis but before ent ...
03-232 Biochemistry
... Choice B: When cellular levels of O2 are limiting during strenuous exercise, glycolysis becomes the main source of energy. Describe what additional step(s) in either yeast or mammalian cells is (are) needed to allow continued utilization of glucose under the low O2 conditions. A. In the cytosol (1pt ...
... Choice B: When cellular levels of O2 are limiting during strenuous exercise, glycolysis becomes the main source of energy. Describe what additional step(s) in either yeast or mammalian cells is (are) needed to allow continued utilization of glucose under the low O2 conditions. A. In the cytosol (1pt ...
KEY - chem.uwec.edu
... though the TCA cannot operate as a cycle in the absence of oxygen (why?), the reactions can be exploited in a way that maintains redox balance. The 4-carbon TCA reactions can run in reverse from oxaloacetate to succinate.. This produces NAD+ and FAD. Simultaneously the cycle runs forward from citrat ...
... though the TCA cannot operate as a cycle in the absence of oxygen (why?), the reactions can be exploited in a way that maintains redox balance. The 4-carbon TCA reactions can run in reverse from oxaloacetate to succinate.. This produces NAD+ and FAD. Simultaneously the cycle runs forward from citrat ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.