Today Electrochemistry electrons moving about equilibrium with a
... Keeping track of charge Easy in ions "Book keeping" in molecules for molecules oxidation numbers are a convention in which we imagine what the charge would be if it broke up into ionic pieces (we can't really assign electrons to different elements) ...
... Keeping track of charge Easy in ions "Book keeping" in molecules for molecules oxidation numbers are a convention in which we imagine what the charge would be if it broke up into ionic pieces (we can't really assign electrons to different elements) ...
Today Electrochemistry electrons moving about equilibrium with a
... Easy in ions! "Book keeping" in molecules! for molecules oxidation numbers are a convention ! in which we imagine what the ! charge would be if it broke up into ionic pieces! (we can't really assign electrons to different elements)! ...
... Easy in ions! "Book keeping" in molecules! for molecules oxidation numbers are a convention ! in which we imagine what the ! charge would be if it broke up into ionic pieces! (we can't really assign electrons to different elements)! ...
H + H–H H∙∙∙∙∙∙∙∙∙ H∙∙∙∙∙∙H H∙∙∙∙∙∙H∙∙∙∙∙∙H
... H + H–H H∙∙∙∙∙∙∙∙∙ H∙∙∙∙∙∙H H∙∙∙∙∙∙H∙∙∙∙∙∙H (activated state) H∙∙∙∙∙∙H∙∙∙∙∙∙∙∙∙∙∙∙H H–H + H This process can be generalized as: A + B-C [ABC] A-B + C Activated complex Transition state ...
... H + H–H H∙∙∙∙∙∙∙∙∙ H∙∙∙∙∙∙H H∙∙∙∙∙∙H∙∙∙∙∙∙H (activated state) H∙∙∙∙∙∙H∙∙∙∙∙∙∙∙∙∙∙∙H H–H + H This process can be generalized as: A + B-C [ABC] A-B + C Activated complex Transition state ...
Rxn Pred students
... These metals can change their oxidation state in a redox reaction Antimony (III) or (V) Bismuth (III) or (IV) Cerium (III) or (IV) Chromium (II) or (III) Cobalt (II) or (III) Copper (I) or (II) Gallium (I) or (II) or (III) Germanium (II) or (IV) Gold (I) or (III) Iron (II) or (II ...
... These metals can change their oxidation state in a redox reaction Antimony (III) or (V) Bismuth (III) or (IV) Cerium (III) or (IV) Chromium (II) or (III) Cobalt (II) or (III) Copper (I) or (II) Gallium (I) or (II) or (III) Germanium (II) or (IV) Gold (I) or (III) Iron (II) or (II ...
Chemistry 1: Second Semester Practice Exam Read each question
... 25. Given the reaction: N2 (g) + 3 H2 (g) Æ 2 NH3 (g). How many liters of ammonia measured at STP are produced when 28.0 grams of nitrogen are completely consumed? A. 5.60 C. 22.4 B. 11.2 D. 44.8 26. What is the Molarity of a solution that contains 112 grams of KOH in 2.00 liters of solution? A. 1.0 ...
... 25. Given the reaction: N2 (g) + 3 H2 (g) Æ 2 NH3 (g). How many liters of ammonia measured at STP are produced when 28.0 grams of nitrogen are completely consumed? A. 5.60 C. 22.4 B. 11.2 D. 44.8 26. What is the Molarity of a solution that contains 112 grams of KOH in 2.00 liters of solution? A. 1.0 ...
Section 4.6: Double Displacement Reactions
... 7. Silver ions are the only metal ions that can be precipitated from a solution containing the C2H3O2− ions. Therefore, a solution such as NaC2H3O2(aq) can be used to precipitate silver ions from a mixture of dissolved metal ions. 8. Answers may vary. Sample answer: Most of the limescale that forms ...
... 7. Silver ions are the only metal ions that can be precipitated from a solution containing the C2H3O2− ions. Therefore, a solution such as NaC2H3O2(aq) can be used to precipitate silver ions from a mixture of dissolved metal ions. 8. Answers may vary. Sample answer: Most of the limescale that forms ...
Multiple-choice questions : 1. The following graph shows the volume
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: ...
... Each question below consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: ...
Chemistr.e1a.chapter.4.new2015
... What are Titrations and How Do We Use Them? A titration is a technique for determining the amount of one chemical present in a sample. It involves a reaction between two chemicals, this reaction can be any type of reaction like a neutralization reaction or a precipitation reaction. In acid-base titr ...
... What are Titrations and How Do We Use Them? A titration is a technique for determining the amount of one chemical present in a sample. It involves a reaction between two chemicals, this reaction can be any type of reaction like a neutralization reaction or a precipitation reaction. In acid-base titr ...
Topic 5 - Chemical Reactions
... Reactions occurring in both forward and reverse directions are reversible. Reversible reactions can reach a state of equilibrium, where reaction rates of the forward and reverse reactions are constant. Le Chatelier’s Principle indicates the qualitative prediction of direction of change with temperat ...
... Reactions occurring in both forward and reverse directions are reversible. Reversible reactions can reach a state of equilibrium, where reaction rates of the forward and reverse reactions are constant. Le Chatelier’s Principle indicates the qualitative prediction of direction of change with temperat ...
Worksheet Key
... 1. Once a system has reached equilibrium, are the following true or false? a. The reaction is finished, no more products are forming. __ false __ b. The concentrations of the reactants and the products are equal. false __ c. The concentrations are no longer changing. _ true _ d. The reaction is not ...
... 1. Once a system has reached equilibrium, are the following true or false? a. The reaction is finished, no more products are forming. __ false __ b. The concentrations of the reactants and the products are equal. false __ c. The concentrations are no longer changing. _ true _ d. The reaction is not ...
Lecture 3
... inserting coefficients before the chemical formulas so that the same number of each type of atom is shown on each side of the equation. Chemical equations may be balances “by inspection” or algebraically (Section 2.1, pages 55-57). Inspection is the preferred way for simple reactions. ...
... inserting coefficients before the chemical formulas so that the same number of each type of atom is shown on each side of the equation. Chemical equations may be balances “by inspection” or algebraically (Section 2.1, pages 55-57). Inspection is the preferred way for simple reactions. ...
chemical reactions
... This is an introduction to chemical reactions. The goal is to demonstrate chemical reactions, reinforce formula writing, introduce students to writing and balancing chemical equations, and to present the reasons why chemical reactions go to completion. This can be reinforced by microscale or small s ...
... This is an introduction to chemical reactions. The goal is to demonstrate chemical reactions, reinforce formula writing, introduce students to writing and balancing chemical equations, and to present the reasons why chemical reactions go to completion. This can be reinforced by microscale or small s ...
Formal balancing of chemical reaction networks
... spanning trees of G directed towards vertex i. In particular, it follows that ρj ≥ 0, j = 1, · · · , c. In fact, ρ 6= 0 if and only if G has a spanning tree. Furthermore, since for every vertex i there exists at least one spanning tree directed towards i if and only if the graph is strongly connecte ...
... spanning trees of G directed towards vertex i. In particular, it follows that ρj ≥ 0, j = 1, · · · , c. In fact, ρ 6= 0 if and only if G has a spanning tree. Furthermore, since for every vertex i there exists at least one spanning tree directed towards i if and only if the graph is strongly connecte ...
Equilibrium STUDY GUIDE by Keshara Senanayake ---
... calculated much like the equilibrium constant except that the concentrations that exist at the time the measurement is taken, not the equilibrium concentrations are inserted into the equilibrium expression. If the reaction is at equilibrium Q would equal Keq But if: Q is less than Keq (Q < Keq), the ...
... calculated much like the equilibrium constant except that the concentrations that exist at the time the measurement is taken, not the equilibrium concentrations are inserted into the equilibrium expression. If the reaction is at equilibrium Q would equal Keq But if: Q is less than Keq (Q < Keq), the ...
[Mg] +2[ S ]-2
... exchange of heat (is it being absorbed or released). State whether the surrounding get cooler or warmer when this type of reaction occurs. Heat is being absorbed in this reaction. The surroundings get cooler during this type of reaction. See Graph A below. ...
... exchange of heat (is it being absorbed or released). State whether the surrounding get cooler or warmer when this type of reaction occurs. Heat is being absorbed in this reaction. The surroundings get cooler during this type of reaction. See Graph A below. ...
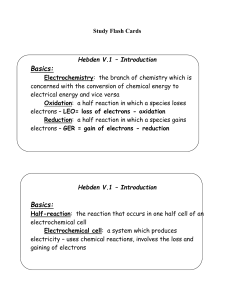
Hebden V.2 – Oxidation Numbers
... the halogens are usually –1 (Cl, Br, I, F) Polyatomic ions have an overall charge that will be shown like OHNeutral molecules do not have a charge shown – it is zero – H4P2O7 has a charge of 0 7. All atoms have charge of 0 8. Hydrogen in all compounds (except hydrides like LiH) is +1 – look to see i ...
... the halogens are usually –1 (Cl, Br, I, F) Polyatomic ions have an overall charge that will be shown like OHNeutral molecules do not have a charge shown – it is zero – H4P2O7 has a charge of 0 7. All atoms have charge of 0 8. Hydrogen in all compounds (except hydrides like LiH) is +1 – look to see i ...
A matter of Equilibrium
... Although rarely include it in the expression for K, instead of using the partial pressure Px of a component we really should use Px/Pref, so that K is then dimensionless. Pref is the standard pressure. This should be 1 bar, but older books (and some new ones) will have used 1 atm. This can make a di ...
... Although rarely include it in the expression for K, instead of using the partial pressure Px of a component we really should use Px/Pref, so that K is then dimensionless. Pref is the standard pressure. This should be 1 bar, but older books (and some new ones) will have used 1 atm. This can make a di ...
2009
... A All the magnesium reacts. B 63.5 g of copper is displaced. C 2 mol of copper is displaced. D The resulting solution is colourless. ...
... A All the magnesium reacts. B 63.5 g of copper is displaced. C 2 mol of copper is displaced. D The resulting solution is colourless. ...




















![[Mg] +2[ S ]-2](http://s1.studyres.com/store/data/014450548_1-468f3af464a09baae245d79fadf97d41-300x300.png)