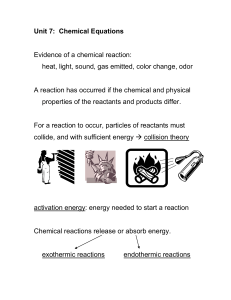
PEA: Chemistry: Mole City Worksheet
... More Mole City 4. How many grams of the aqueous product would be formed in double replacement reaction when 10.0 grams of aqueous barium chloride is reacted with an excess amount of aqueous silver nitrate. ...
... More Mole City 4. How many grams of the aqueous product would be formed in double replacement reaction when 10.0 grams of aqueous barium chloride is reacted with an excess amount of aqueous silver nitrate. ...
CHEM 150
... ____ 26. Which of the following affects the vapor pressure of a liquid? a. the mass of the sample b. the shape the sample c. the temperature of the sample d. the volume of the sample ____ 27. Chloroform has a normal boiling point of 61.7oC. Which of the following is true? a. at any temperature the ...
... ____ 26. Which of the following affects the vapor pressure of a liquid? a. the mass of the sample b. the shape the sample c. the temperature of the sample d. the volume of the sample ____ 27. Chloroform has a normal boiling point of 61.7oC. Which of the following is true? a. at any temperature the ...
nomenclature review
... S2Cl2(g) + CCl4(g) When 2.14 mol of CS2 and 5.85 mol of Cl2 are placed in a 2.00-L container and allowed to come to equilibrium, the mixture is found to contain 0.620 mol of CCl4. How many moles of Cl2 are present at equilibrium? (3.99 mol) ...
... S2Cl2(g) + CCl4(g) When 2.14 mol of CS2 and 5.85 mol of Cl2 are placed in a 2.00-L container and allowed to come to equilibrium, the mixture is found to contain 0.620 mol of CCl4. How many moles of Cl2 are present at equilibrium? (3.99 mol) ...
AP Chem Chapter 16 Review Packet
... a. Calculate H, S, and G for both reactions at 25C. b. Write a balanced equation for the overall reaction which occurs on summing these two equations. What law is illustrated by this process? What is the enthalpy change, H, for the two reactions added together? c. What is the G for the tw ...
... a. Calculate H, S, and G for both reactions at 25C. b. Write a balanced equation for the overall reaction which occurs on summing these two equations. What law is illustrated by this process? What is the enthalpy change, H, for the two reactions added together? c. What is the G for the tw ...
Chemistry - SchoolNotes.com
... b) silver sulfide 87% Ag, 13%S d) Sr(CH3COO)2 Sr=43% C=23% H=3% O31 7) What is a hydrate? A compound in which the ions are attached to one or more water molecules. 8) How does the empirical formula differ from the molecular formula? Empirical formula is the molecular formula in the lowest ratio 9) C ...
... b) silver sulfide 87% Ag, 13%S d) Sr(CH3COO)2 Sr=43% C=23% H=3% O31 7) What is a hydrate? A compound in which the ions are attached to one or more water molecules. 8) How does the empirical formula differ from the molecular formula? Empirical formula is the molecular formula in the lowest ratio 9) C ...
Equations - Pearson Schools and FE Colleges
... 2Li + H2SO4 → Li2SO4 + H2 Check that each formula is on the correct side of the equation, and then count the atoms on the table. each side of the equation to see if this equation is balanced. Complete Reactants side of equation ...
... 2Li + H2SO4 → Li2SO4 + H2 Check that each formula is on the correct side of the equation, and then count the atoms on the table. each side of the equation to see if this equation is balanced. Complete Reactants side of equation ...
Exam 3 Review Key
... (this is for basic conditions, used because this is how the reaction is given in the Standard Potentials Table in the book’s appendix. In acid, the cell would have the same set-up, only there would be H+ on the right and no OH- on the left) 12. Calculate the reaction quotient, Q, for the cell reacti ...
... (this is for basic conditions, used because this is how the reaction is given in the Standard Potentials Table in the book’s appendix. In acid, the cell would have the same set-up, only there would be H+ on the right and no OH- on the left) 12. Calculate the reaction quotient, Q, for the cell reacti ...
Test
... 25 Which of these compounds has chemical properties most similar to the chemical properties of ethanoic acid? (1) C3H7COOH (3) C2H5COOC2H5 (2) C2H5OH (4) C2H5OC2H5 ...
... 25 Which of these compounds has chemical properties most similar to the chemical properties of ethanoic acid? (1) C3H7COOH (3) C2H5COOC2H5 (2) C2H5OH (4) C2H5OC2H5 ...
Export To Word
... This lesson requires the students to use their observation skills and their knowledge of single-replacement and double-replacement The Mystery of the Chemistry reactions to solve a mystery. The students will be performing a Lab Explosion laboratory experiment to solve the mystery; therefore, groups ...
... This lesson requires the students to use their observation skills and their knowledge of single-replacement and double-replacement The Mystery of the Chemistry reactions to solve a mystery. The students will be performing a Lab Explosion laboratory experiment to solve the mystery; therefore, groups ...
ASFG High School Summer Assignment Summer 2016
... b.Epinephrine( adrenaline) a hormone secreted into the bloodstream in times of danger or stress contains 59% C, 7.1% H, 26.2% O, and 7.7% N by mass, its MW is about 180 amu. 52.Write balanced chemical equations for the reactions of sodium with the following nonmetals to form ionic solids. a ...
... b.Epinephrine( adrenaline) a hormone secreted into the bloodstream in times of danger or stress contains 59% C, 7.1% H, 26.2% O, and 7.7% N by mass, its MW is about 180 amu. 52.Write balanced chemical equations for the reactions of sodium with the following nonmetals to form ionic solids. a ...
Moles and Stoichiometry - Ms. Randall`s Science Scene
... • A substance that SPEEDS UP a reaction • Written ABOVE the arrow • Example: (elephants toothpaste) KI 2 H2O2(aq) -----> 2 H2O(l) + O2(g) ...
... • A substance that SPEEDS UP a reaction • Written ABOVE the arrow • Example: (elephants toothpaste) KI 2 H2O2(aq) -----> 2 H2O(l) + O2(g) ...
Balancing and Predicting Chemical Reactions:
... a. 3 Li(s) + Fe(NO3)3(aq) 3 LiNO3(aq) + Fe(s) b. Au(s) + HCl(aq) NR c. Cl2(g) + 2 KBr(aq) 2 KCl(aq) + Br2(l) d. Cu(s) + Al(NO3)3(aq) NR e. Ag(s) + HBr(aq) NR f. Ni(s) + SnCl2(aq) Sn(s) + NiCl2(aq) ...
... a. 3 Li(s) + Fe(NO3)3(aq) 3 LiNO3(aq) + Fe(s) b. Au(s) + HCl(aq) NR c. Cl2(g) + 2 KBr(aq) 2 KCl(aq) + Br2(l) d. Cu(s) + Al(NO3)3(aq) NR e. Ag(s) + HBr(aq) NR f. Ni(s) + SnCl2(aq) Sn(s) + NiCl2(aq) ...
C4C5C6
... If the equilibrium is to the left then there is more reactant and not so much product. ...
... If the equilibrium is to the left then there is more reactant and not so much product. ...
Chemistry Name Mr. Reger Review Guide – Ch. 9
... 2 Al + 3 Br2 2 AlBr3 How many moles of AlBr3 are expected to form? a) 2.0 b) 3.0 c) 4.0 d) 6.0 e) 8.0 ...
... 2 Al + 3 Br2 2 AlBr3 How many moles of AlBr3 are expected to form? a) 2.0 b) 3.0 c) 4.0 d) 6.0 e) 8.0 ...