percent composition and formulas
... left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
... left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
2016
... platinum. If 154 g of gold are available, how many grams of platinum are required to combine with the gold to form this alloy? 20.What is the empirical formula of a compound that contains 53.73% Fe and 46.27% of S ? 21.Determine the number of molecules present in 4.50 mol of Nitrogen dioxide, the nu ...
... platinum. If 154 g of gold are available, how many grams of platinum are required to combine with the gold to form this alloy? 20.What is the empirical formula of a compound that contains 53.73% Fe and 46.27% of S ? 21.Determine the number of molecules present in 4.50 mol of Nitrogen dioxide, the nu ...
Chapter 3 Chemical Reactions and Reaction Stoichiometry
... Ø e.g. On the previous slide, the theoretical yield is 10 H2O molecules. Ø A theoretical yield calculation must be performed whenever definite amounts of more than one reactant are given. ...
... Ø e.g. On the previous slide, the theoretical yield is 10 H2O molecules. Ø A theoretical yield calculation must be performed whenever definite amounts of more than one reactant are given. ...
Potassium Bromate as an Oxidizing Agent in a
... function of the KBrO3 concentration at pH 2. The etch rate is approximately proportional to the square root of the KBrO3 concentration, implying that Ru dissolution is a multistep process with an intermediate species participating in a second-order reaction. The etch rates are much lower than the CM ...
... function of the KBrO3 concentration at pH 2. The etch rate is approximately proportional to the square root of the KBrO3 concentration, implying that Ru dissolution is a multistep process with an intermediate species participating in a second-order reaction. The etch rates are much lower than the CM ...
Chapter 7. CHEMICAL REACTIONS
... The law of Conservation of Matter (or mass) states that during a chemical reaction the total mass of reactants is the same as the total mass of products formed. During a ...
... The law of Conservation of Matter (or mass) states that during a chemical reaction the total mass of reactants is the same as the total mass of products formed. During a ...
CHAPTER 9
... (1) The only information needed to write an equilibrium constant expression is a balanced chemical equation with physical state information for the reaction of concern. (2) The oxidation number of sulfur is the same in the species H2SO4 and SO42-. (3) Increasing the concentration of a reactant makes ...
... (1) The only information needed to write an equilibrium constant expression is a balanced chemical equation with physical state information for the reaction of concern. (2) The oxidation number of sulfur is the same in the species H2SO4 and SO42-. (3) Increasing the concentration of a reactant makes ...
PowerPoint Lectures - Northwest ISD Moodle
... reaction mixture (i.e., solid, liquid, gas, aqueous solution). • If we are to understand reactivity, we must be aware of just what is changing during the course of a reaction… the driving force! – The driving force is what makes the reaction react (Ex: formation of a precipitate, gas, or liquid) – W ...
... reaction mixture (i.e., solid, liquid, gas, aqueous solution). • If we are to understand reactivity, we must be aware of just what is changing during the course of a reaction… the driving force! – The driving force is what makes the reaction react (Ex: formation of a precipitate, gas, or liquid) – W ...
Word - chemmybear.com
... 40. For this reaction, E°cell = 0.79 V. 6I¯(aq) + Cr2O72¯(aq) + 14H+ 3I2 (aq) + 2Cr3+(aq) + 7H2O(aq) Given that the standard reduction potential for Cr2O72¯(aq) 2Cr3+ (aq) is 1.33 V, what is E°red for I2(aq)? a) +0.54 V b) -0.54 V c) +0.18 V d) -0.18 V ...
... 40. For this reaction, E°cell = 0.79 V. 6I¯(aq) + Cr2O72¯(aq) + 14H+ 3I2 (aq) + 2Cr3+(aq) + 7H2O(aq) Given that the standard reduction potential for Cr2O72¯(aq) 2Cr3+ (aq) is 1.33 V, what is E°red for I2(aq)? a) +0.54 V b) -0.54 V c) +0.18 V d) -0.18 V ...
111 Exam I Outline
... Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
... Key: You must have a balanced equation!! How many grams of chromic chloride reacts with 6.0 mole Cr? ...
Chapter 1: Fundamental Concepts
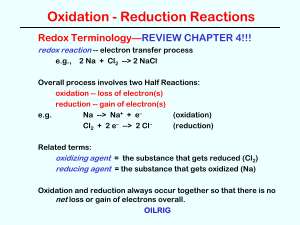
... 1. Write unbalanced ionic equations for the two half-reactions. (Look at oxidation numbers in the “skeleton equation.”) 2. Balance atoms other than H and O. 3. Add appropriate number of electrons. 4. Balance O with H2O. 5. Balance H with H+. 6. If in acidic solution, then skip to step 7. If in basic ...
... 1. Write unbalanced ionic equations for the two half-reactions. (Look at oxidation numbers in the “skeleton equation.”) 2. Balance atoms other than H and O. 3. Add appropriate number of electrons. 4. Balance O with H2O. 5. Balance H with H+. 6. If in acidic solution, then skip to step 7. If in basic ...
Topic 1: Quantitative Chemistry
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
Section A oxide in molten cryolite?
... Q16 Sodium iodide reacts with concentrated sulfuric acid. The equation which represents one of the reactions that takes place is shown. 8NaI + 9H2SO4 → 8NaHSO4 + 4I2 + H2S + 4H2O Which species has been oxidised in this reaction? AH+ B I– C Na+ DSO42– Q17 In which reaction does an element undergo the ...
... Q16 Sodium iodide reacts with concentrated sulfuric acid. The equation which represents one of the reactions that takes place is shown. 8NaI + 9H2SO4 → 8NaHSO4 + 4I2 + H2S + 4H2O Which species has been oxidised in this reaction? AH+ B I– C Na+ DSO42– Q17 In which reaction does an element undergo the ...
Step by Step Stoichiometry
... As you can see I’m limited in making a complete Mr. Potato Head by the parts available to me. Even though I have 15 parts, I can only make 1 Mr. Potato Had in the first example, leaving 3 parts in unused or in excess. ...
... As you can see I’m limited in making a complete Mr. Potato Head by the parts available to me. Even though I have 15 parts, I can only make 1 Mr. Potato Had in the first example, leaving 3 parts in unused or in excess. ...