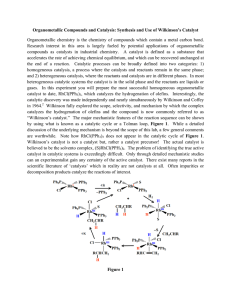
Types of Chemical Reactions (rxns.)
... Some steps for doing reactions: 1. Identify the type of reaction 2. Predict the product(s) using the type of reaction as a ...
... Some steps for doing reactions: 1. Identify the type of reaction 2. Predict the product(s) using the type of reaction as a ...
3(aq)
... 1. Strong acids dissolve completely into H+ ions and an anion. (ex: HCL, HNO3, and H2SO4) 2. Strong bases (metal hydroxides) will dissolve completely into OH- ions and a metallic cation. (ex: NaOH and KOH) 3. The net ionic equation for a reaction between a base and an acid is: H+(aq) + OH-(aq) H2O ...
... 1. Strong acids dissolve completely into H+ ions and an anion. (ex: HCL, HNO3, and H2SO4) 2. Strong bases (metal hydroxides) will dissolve completely into OH- ions and a metallic cation. (ex: NaOH and KOH) 3. The net ionic equation for a reaction between a base and an acid is: H+(aq) + OH-(aq) H2O ...
Unit 3 Notes
... When a reaction takes place between 2 reactants, it is very unlikely that both of the substances are in exactly the right proportions and that they will both run out at the same time. Usually, one runs out before the other and this reactant limits how much product can be formed. The reaction will be ...
... When a reaction takes place between 2 reactants, it is very unlikely that both of the substances are in exactly the right proportions and that they will both run out at the same time. Usually, one runs out before the other and this reactant limits how much product can be formed. The reaction will be ...
Limiting Reactants and Percent Yield
... Draw in the NH3 molecules that will result from the reaction of hydrogen and nitrogen in the above container. How many NH3 molecules will be formed? _______________________ What will be left over?__________________________ To determine how much product will be formed from a given mixture of reactant ...
... Draw in the NH3 molecules that will result from the reaction of hydrogen and nitrogen in the above container. How many NH3 molecules will be formed? _______________________ What will be left over?__________________________ To determine how much product will be formed from a given mixture of reactant ...
Exam 1
... The best description of the effect of a catalyst on a chemical reaction is that it A. lowers the activation energy of the forward reaction without changing the activation energy of the reverse reaction. B. lowers the activation energy of the forward reaction and raises the activation energy of the r ...
... The best description of the effect of a catalyst on a chemical reaction is that it A. lowers the activation energy of the forward reaction without changing the activation energy of the reverse reaction. B. lowers the activation energy of the forward reaction and raises the activation energy of the r ...
pdf version - Joliet Junior College
... Review: What follows is a recap of the most important topics covered in CHM 101. We will use this material throughout CHM 102, so please ensure that you are familiar with the following questions, as well as the Ch3 & 4 HWK questions, before we move on to the Ch 11 material. Top Tip: Committing to a ...
... Review: What follows is a recap of the most important topics covered in CHM 101. We will use this material throughout CHM 102, so please ensure that you are familiar with the following questions, as well as the Ch3 & 4 HWK questions, before we move on to the Ch 11 material. Top Tip: Committing to a ...
Review Packet - Newton.k12.ma.us
... - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quantity will be much smaller and a number that is easier to deal with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams ...
... - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quantity will be much smaller and a number that is easier to deal with than if you use grams or pounds. Also, you can compare two quantities of moles to each other, but you cannot compare grams ...
AP Chemistry Summer Assignment
... best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources available via the Internet. With the ready access to hundreds of websites either in your home or at the local library, I am confident t ...
... best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources available via the Internet. With the ready access to hundreds of websites either in your home or at the local library, I am confident t ...
Reactants Products
... In the first 10.0 seconds of the reaction, the concentration of I– dropped from 1.000 M to 0.868 M. a. Calculate the average rate of this reaction in this time interval. b. Determine the rate of change in the concentration of H+ (that is, Δ[H+]/Δt) during this time interval. ...
... In the first 10.0 seconds of the reaction, the concentration of I– dropped from 1.000 M to 0.868 M. a. Calculate the average rate of this reaction in this time interval. b. Determine the rate of change in the concentration of H+ (that is, Δ[H+]/Δt) during this time interval. ...
50 Frequently Forgotten Facts Answer Key
... 26) Molecular compounds tend to be soft, have low melting points and high vapor pressures. Hydrogen bonds are the strongest of the intermolecular forces (when the H of one polar molecule attracts the N, O or F of another polar molecule), followed by dipole (where the more electronegative end of one ...
... 26) Molecular compounds tend to be soft, have low melting points and high vapor pressures. Hydrogen bonds are the strongest of the intermolecular forces (when the H of one polar molecule attracts the N, O or F of another polar molecule), followed by dipole (where the more electronegative end of one ...