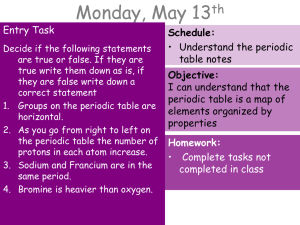
Entry Task
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
Chapter 1 Chemistry: The Study of Matter
... Elements- simplest kind of matter, made of one type of atom An atom is the smallest unit of an element that maintains the properties of that element. Cannot be broken down into simpler substances by ordinary chemical means Ex. gold, copper, oxygen (on the periodic table) ...
... Elements- simplest kind of matter, made of one type of atom An atom is the smallest unit of an element that maintains the properties of that element. Cannot be broken down into simpler substances by ordinary chemical means Ex. gold, copper, oxygen (on the periodic table) ...
Worksheet
... Which of the following is true regarding the reaction represented above? (A) The oxidation number of O does not change. (B) The oxidation number of H changes from -1 to +1. (C) The oxidation number of F changes from +1 to -1. (D) The oxidation number of Se changes from -2 to +6. (E) It is a dispropo ...
... Which of the following is true regarding the reaction represented above? (A) The oxidation number of O does not change. (B) The oxidation number of H changes from -1 to +1. (C) The oxidation number of F changes from +1 to -1. (D) The oxidation number of Se changes from -2 to +6. (E) It is a dispropo ...
7.1 Describing Reactions
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
7.1 Describing Reactions
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
7.1 Describing Reactions
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
Slide 1
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
... To calculate how much oxygen is required to make 144 grams of water, begin with a balanced chemical equation for the reaction. 2H2 + O2 2H2O • Determine how many moles of water you are trying to ...
1 Quantitative chemistry - Pearson Schools and FE Colleges
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
Chapter 19 CHEMICAL THERMODYNAMICS 19.1 SPONTANEOUS
... the number of possible microstates increases and so does the entropy. In water vapor, the molecules are essentially independent of one another and have their full range of translational, vibrational, and rotational motions. Thus, water vapor has an even greater number of possible microstates and the ...
... the number of possible microstates increases and so does the entropy. In water vapor, the molecules are essentially independent of one another and have their full range of translational, vibrational, and rotational motions. Thus, water vapor has an even greater number of possible microstates and the ...
Chemistry Standard Level Chapter 1
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
classical damping constant
... Natural Broadening • From Heisenberg's uncertainty principle: The electron in an excited state is only there for a short time, so its energy cannot have a precise value. • Since energy levels are "fuzzy," atoms can absorb photons with slightly different energy, with the probability of absorption de ...
... Natural Broadening • From Heisenberg's uncertainty principle: The electron in an excited state is only there for a short time, so its energy cannot have a precise value. • Since energy levels are "fuzzy," atoms can absorb photons with slightly different energy, with the probability of absorption de ...
Chemistry I
... 100 g of carbon reacts with 133 g oxygen to carbon monoxide 100 g of carbon reacts with 266 g oxygen to carbon dioxide ...
... 100 g of carbon reacts with 133 g oxygen to carbon monoxide 100 g of carbon reacts with 266 g oxygen to carbon dioxide ...
I. The Atomic Concept:
... He concluded that the deflected alpha particles must have encountered positively charged centers in the gold foil, which deflect them from a straight course due to like-charge repulsion. A few alpha particles may have collided head-on with these centers, which must be dense or massive enough to boun ...
... He concluded that the deflected alpha particles must have encountered positively charged centers in the gold foil, which deflect them from a straight course due to like-charge repulsion. A few alpha particles may have collided head-on with these centers, which must be dense or massive enough to boun ...
2.3 Atomic Mass and Number
... one proton, we know it’s an atom of the element hydrogen. An atom with two protons is always an atom of the element helium. When scientists count four protons in an atom, they know it’s a beryllium atom. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom ...
... one proton, we know it’s an atom of the element hydrogen. An atom with two protons is always an atom of the element helium. When scientists count four protons in an atom, they know it’s a beryllium atom. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom ...
CHM 312
... Ferromagnetism: A ferromagnetic substance is one that, like iron, retains a magnetic moment even when the external magnetic field is reduced to zero. This effect is a result of a strong interaction between the magnetic moments of the individual atoms or electrons in the magnetic substance that cause ...
... Ferromagnetism: A ferromagnetic substance is one that, like iron, retains a magnetic moment even when the external magnetic field is reduced to zero. This effect is a result of a strong interaction between the magnetic moments of the individual atoms or electrons in the magnetic substance that cause ...
Reduction and Emergence in Chemistry
... Admittedly the quantum mechanical theory of bonding that McLaughlin appeals to does, provide a more fundamental account of chemical bonding than the classical, or Lewis theory. Nevertheless, it does not permit one to predict in advance the behavior of elements or the properties that a compound might ...
... Admittedly the quantum mechanical theory of bonding that McLaughlin appeals to does, provide a more fundamental account of chemical bonding than the classical, or Lewis theory. Nevertheless, it does not permit one to predict in advance the behavior of elements or the properties that a compound might ...
Reduction and Emergence in Chemistry - Philsci
... Admittedly the quantum mechanical theory of bonding that McLaughlin appeals to does, provide a more fundamental account of chemical bonding that the classical, or Lewis theory. Nevertheless, it does not permit one to predict in advance the behavior of elements or the properties that a compound might ...
... Admittedly the quantum mechanical theory of bonding that McLaughlin appeals to does, provide a more fundamental account of chemical bonding that the classical, or Lewis theory. Nevertheless, it does not permit one to predict in advance the behavior of elements or the properties that a compound might ...
2.0 Chem 20 Final Review
... • So why do we care about bonding capacity? – If we know how many bonding e-’s an atom has, we can predict what structure a molecular compound will have Atom ...
... • So why do we care about bonding capacity? – If we know how many bonding e-’s an atom has, we can predict what structure a molecular compound will have Atom ...
Chemistry
... protons, electrons and neutrons.[4] Atoms combine to produce molecules or crystals. Chemistry is sometimes called "the central science" because it connects the other natural sciences such as astronomy, physics, material science, biology and geology.[5] [6] The genesis of chemistry can be traced to c ...
... protons, electrons and neutrons.[4] Atoms combine to produce molecules or crystals. Chemistry is sometimes called "the central science" because it connects the other natural sciences such as astronomy, physics, material science, biology and geology.[5] [6] The genesis of chemistry can be traced to c ...
Atomic structure
... ‘All things are made from atoms.’ This is one of the most important ideas that the human race has learned about the universe. Atoms are everywhere and they make up everything. You are surrounded by atoms – they make up the foods you eat, the liquids you drink, and the fragrances you smell. Atoms mak ...
... ‘All things are made from atoms.’ This is one of the most important ideas that the human race has learned about the universe. Atoms are everywhere and they make up everything. You are surrounded by atoms – they make up the foods you eat, the liquids you drink, and the fragrances you smell. Atoms mak ...
Chapter 2 Tro Chemistry - Highline Community College
... Practice – Decide if each statement is correct according to Dalton’s model of the atom • Copper atoms can combine with zinc atoms to make gold atoms – incorrect; according to Dalton, atoms of one element cannot turn into atoms of another element by a chemical reaction. He knew this because if atoms ...
... Practice – Decide if each statement is correct according to Dalton’s model of the atom • Copper atoms can combine with zinc atoms to make gold atoms – incorrect; according to Dalton, atoms of one element cannot turn into atoms of another element by a chemical reaction. He knew this because if atoms ...
c = λv
... Lesson Starter • The electron configuration of carbon is 1s22s22p2. • An electron configuration describes the arrangement of electrons in an atom. • The integers indicate the main energy level of each orbital occupied by electrons. • The letters indicate the shape of the occupied ...
... Lesson Starter • The electron configuration of carbon is 1s22s22p2. • An electron configuration describes the arrangement of electrons in an atom. • The integers indicate the main energy level of each orbital occupied by electrons. • The letters indicate the shape of the occupied ...
mole
... • Obtain lowest whole-number ratio by multiplying each part of the ratio by the smallest whole number that will convert both subscripts into whole numbers 1 mol N X 2 = 2 mol N 2.5 mol O X 2 = 5 mol O Empirical Formula = N2O5 ...
... • Obtain lowest whole-number ratio by multiplying each part of the ratio by the smallest whole number that will convert both subscripts into whole numbers 1 mol N X 2 = 2 mol N 2.5 mol O X 2 = 5 mol O Empirical Formula = N2O5 ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.