Chapter 4
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...
Learning about atoms
... The atom makes up everything around us. It is the building block of all matter (any solid, liquid, or gas). The word atom means “indivisible” so it is the smallest particle of all matter. It is so small that we cannot see it with our eye. In fact, it is so small that it has been said that more than ...
... The atom makes up everything around us. It is the building block of all matter (any solid, liquid, or gas). The word atom means “indivisible” so it is the smallest particle of all matter. It is so small that we cannot see it with our eye. In fact, it is so small that it has been said that more than ...
Chemistry II Honors – Unit 3 Study Guide
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
... Iron is biologically important in the transport of oxygen by red blood cells from the lungs to the various organs of the body. In the blood of an adult human, there are approximately 2.65e13 red blood cells with a total of 2.90 g of iron. On the average, how many iron atoms are present in each red b ...
Preface from the Textbook - McGraw Hill Higher Education
... spectra (the specific colors emitted from a substance that is excited)—can only be Light from Excited Atoms In a fireworks display and explained if energy consists of “packets” (quanta) that occur in, and thus change by, fixed amounts. The energy of a quantum is related to its frequency. many other ...
... spectra (the specific colors emitted from a substance that is excited)—can only be Light from Excited Atoms In a fireworks display and explained if energy consists of “packets” (quanta) that occur in, and thus change by, fixed amounts. The energy of a quantum is related to its frequency. many other ...
Lecture 7
... Their atoms can lose the two outer electrons to form a cation with a charge of +2. Because the outer electrons are far from the nucleus and easily lost, they are all strong reducing agents. Going down the group the outer electrons get further from the nucleus and so are more weakly held. This is bec ...
... Their atoms can lose the two outer electrons to form a cation with a charge of +2. Because the outer electrons are far from the nucleus and easily lost, they are all strong reducing agents. Going down the group the outer electrons get further from the nucleus and so are more weakly held. This is bec ...
1 Structure of Atom - Viva Online Learning
... particles called paramanu (param means ultimate and anu means particle). A paramanu does not exist in free state, rather it combines with other paramanus to form a bigger particle called the anu. In other words, he believed that an anu may be made up of two or more subatomic particles called paraman ...
... particles called paramanu (param means ultimate and anu means particle). A paramanu does not exist in free state, rather it combines with other paramanus to form a bigger particle called the anu. In other words, he believed that an anu may be made up of two or more subatomic particles called paraman ...
Web Quest and Poster Project Guidelines
... had been observed for hydrogen. Remember, as the website informs us, the only lines that we can see (visible light) are between 440 and 600nM. Just a sliver of the spectrum. Another thing to remember is that if electrons could be found in orbitals at any distance, the spectrum would not be broken in ...
... had been observed for hydrogen. Remember, as the website informs us, the only lines that we can see (visible light) are between 440 and 600nM. Just a sliver of the spectrum. Another thing to remember is that if electrons could be found in orbitals at any distance, the spectrum would not be broken in ...
Webquest and Project Guidelines
... had been observed for hydrogen. Remember, as the website informs us, the only lines that we can see (visible light) are between 440 and 600nM. Just a sliver of the spectrum. Another thing to remember is that if electrons could be found in orbitals at any distance, the spectrum would not be broken in ...
... had been observed for hydrogen. Remember, as the website informs us, the only lines that we can see (visible light) are between 440 and 600nM. Just a sliver of the spectrum. Another thing to remember is that if electrons could be found in orbitals at any distance, the spectrum would not be broken in ...
Advanced Placement Chemistry
... listed in the table above. On the basis of the data, element X is most likely to be (A) Na (B) Mg (C) Al (D) Si (E) P 38. A molecule or an ion is classified as a Lewis acid if it (A) accepts a proton from water (B) accepts a pair of electrons to form a bond (C) donates a pair of electrons to form a ...
... listed in the table above. On the basis of the data, element X is most likely to be (A) Na (B) Mg (C) Al (D) Si (E) P 38. A molecule or an ion is classified as a Lewis acid if it (A) accepts a proton from water (B) accepts a pair of electrons to form a bond (C) donates a pair of electrons to form a ...
DEPARTMENT OF CHEMISTRY, CFS, IIUM
... variety of matter is recognized is called a property. A characteristic that depends upon the amount of matter in the sample is called an extensive property. A characteristic that does not depend upon the amount of matter is called an intensive property. A characteristic that can be observed without ...
... variety of matter is recognized is called a property. A characteristic that depends upon the amount of matter in the sample is called an extensive property. A characteristic that does not depend upon the amount of matter is called an intensive property. A characteristic that can be observed without ...
File - Mrs. Dawson`s Classroom
... The standard used to compare units of atomic mass is the carbon- 12 atom. It has been arbitrarily assigned a mass of exactly 12 atomic mass units or 12 amu One atomic mass unit (amu) is exactly 1/12 the mass of a carbon 12 atom ...
... The standard used to compare units of atomic mass is the carbon- 12 atom. It has been arbitrarily assigned a mass of exactly 12 atomic mass units or 12 amu One atomic mass unit (amu) is exactly 1/12 the mass of a carbon 12 atom ...
Head-Gordon`s
... However, it has been known for three decades that the exact energy is in fact a functional of only the electron density (a function of only 3, rather than 3n, variables). The only catch is that the functional is not known. In section 4, I discuss density functional theory (DFT) which focuses on the ...
... However, it has been known for three decades that the exact energy is in fact a functional of only the electron density (a function of only 3, rather than 3n, variables). The only catch is that the functional is not known. In section 4, I discuss density functional theory (DFT) which focuses on the ...
Document
... (c) Which point of Dalton’s atomic theory is based on the law of constant proportion proposed by Proust in 1799 which states that all pure samples of the same chemical compound contain the same elements combined together in the same proportions by mass? (c) Atoms of different elements combine to for ...
... (c) Which point of Dalton’s atomic theory is based on the law of constant proportion proposed by Proust in 1799 which states that all pure samples of the same chemical compound contain the same elements combined together in the same proportions by mass? (c) Atoms of different elements combine to for ...
225 Unit 7, Lab 1 - Pope John Paul II High School
... Excess reactants will not be consumed. In the example seen above, 3O2 had to be added to the right side of the equation to balance it and show that the excess oxygen is not consumed during the reaction. In this example, methane is called the limiting reactant. Although we have discussed balancing eq ...
... Excess reactants will not be consumed. In the example seen above, 3O2 had to be added to the right side of the equation to balance it and show that the excess oxygen is not consumed during the reaction. In this example, methane is called the limiting reactant. Although we have discussed balancing eq ...
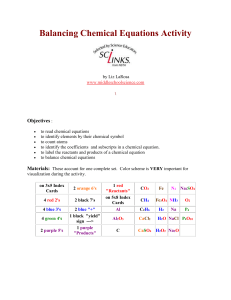
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
Microsoft Word
... All acetates are soluble except for Be(CH3COO)2 All phosphates are insoluble except for those of Group I elements and NH4+. All carbonates are insoluble except for those of Group I elements and NH4+. All hydroxides are insoluble except for those of NH4+, Group I, Sr(OH)2, and Ba(OH)2; Ca(OH)2 is sli ...
... All acetates are soluble except for Be(CH3COO)2 All phosphates are insoluble except for those of Group I elements and NH4+. All carbonates are insoluble except for those of Group I elements and NH4+. All hydroxides are insoluble except for those of NH4+, Group I, Sr(OH)2, and Ba(OH)2; Ca(OH)2 is sli ...
Advanced Higher Chemistry Resource Guide
... number of wavelengths. The principal quantum number, n, is the circumference of the orbital in terms of the number of wavelengths. Revised Higher 2(a) atomic orbital notes page 28. The RSC website has pages which offer very clear and attractive representations of orbitals with accompanying text whic ...
... number of wavelengths. The principal quantum number, n, is the circumference of the orbital in terms of the number of wavelengths. Revised Higher 2(a) atomic orbital notes page 28. The RSC website has pages which offer very clear and attractive representations of orbitals with accompanying text whic ...
Final Exam Review
... 60. A molecule in which the central atom has no lone pairs and forms four single bonds is said to have a _________________ shape. a. bent b. linear c. planar d. pyramidal e. tetrahedral 61. The water molecule has a _______ geometry because its central atom has _____ bonds and _____ lone pairs of el ...
... 60. A molecule in which the central atom has no lone pairs and forms four single bonds is said to have a _________________ shape. a. bent b. linear c. planar d. pyramidal e. tetrahedral 61. The water molecule has a _______ geometry because its central atom has _____ bonds and _____ lone pairs of el ...
atomic theory - Scorpion Science with Mrs. Page
... 1.Atomic structure: subatomic particles, their charges, location, & mass. 2.How periodic table was organized & how chemists changed it to what we use today. 3.Organization of periodic table: groups & periods, reactivity of metals & nonmetals. ...
... 1.Atomic structure: subatomic particles, their charges, location, & mass. 2.How periodic table was organized & how chemists changed it to what we use today. 3.Organization of periodic table: groups & periods, reactivity of metals & nonmetals. ...
PHYSICAL SETTING CHEMISTRY
... Base your answers to questions 73 through 76 on the information below. A portable propane-fueled lantern contains a mesh silk bag coated with metal hydroxides. The primary metal hydroxide is yttrium hydroxide. When the silk bag is installed, it is ignited and burned away, leaving the metal hydroxid ...
... Base your answers to questions 73 through 76 on the information below. A portable propane-fueled lantern contains a mesh silk bag coated with metal hydroxides. The primary metal hydroxide is yttrium hydroxide. When the silk bag is installed, it is ignited and burned away, leaving the metal hydroxid ...
Chemistry - Birkenhead School
... 2.2.1 The three states of matter Content Opportunities for skills development The three states of matter are solid, liquid and gas. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling point. The three states of matter can be represented by a simple ...
... 2.2.1 The three states of matter Content Opportunities for skills development The three states of matter are solid, liquid and gas. Melting and freezing take place at the melting point, boiling and condensing take place at the boiling point. The three states of matter can be represented by a simple ...
View PDF
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
l-Changing Collisions in Cold Rydberg Gases
... dependence of this long-lasting electron signal is shown in Fig. 1. There, the dye laser was operated at a low pulse energy (< 1 mJ) and at fixed wavelengths. The similarity of the two depicted results is coincidental. The absolute strength of the signal has been found to depend on the quantum numbe ...
... dependence of this long-lasting electron signal is shown in Fig. 1. There, the dye laser was operated at a low pulse energy (< 1 mJ) and at fixed wavelengths. The similarity of the two depicted results is coincidental. The absolute strength of the signal has been found to depend on the quantum numbe ...
8872 Chemistry H1 syllabus for 2016
... (a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium (b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, ...
... (a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium (b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.