Materials Required
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
Problem Solving Drill - Rapid Learning Center
... cannot have different number of protons. Atoms of the same element with different numbers of neutrons are called isotopes. The number of electrons can change to form atoms with a charge. The correct answer is (D). ...
... cannot have different number of protons. Atoms of the same element with different numbers of neutrons are called isotopes. The number of electrons can change to form atoms with a charge. The correct answer is (D). ...
Problem Solving Drill - Rapid Learning Center
... cannot have different number of protons. Atoms of the same element with different numbers of neutrons are called isotopes. The number of electrons can change to form atoms with a charge. The correct answer is (D). ...
... cannot have different number of protons. Atoms of the same element with different numbers of neutrons are called isotopes. The number of electrons can change to form atoms with a charge. The correct answer is (D). ...
CHEMISTRY 101 Name Mock Final Exam Spring 2014 Signature Dr
... a) The most positively charged species has the largest atomic radius, and the most negatively charged species has the smallest atomic radius. b) The noble gas has the largest atomic radius, and the most negatively charged species has the smallest atomic radius. c) The noble gas has the largest atomi ...
... a) The most positively charged species has the largest atomic radius, and the most negatively charged species has the smallest atomic radius. b) The noble gas has the largest atomic radius, and the most negatively charged species has the smallest atomic radius. c) The noble gas has the largest atomi ...
CHEMISTRY Test 3: Atomic Structure
... ____ 38. The spin quantum number indicates that the number of possible states for an electron in an orbital is a. 1. c. 3. b. 2. d. 5. ____ 39. The spin quantum number of an electron can be thought of as describing a. the direction of electron spin. b. whether the electron's charge is positive or ne ...
... ____ 38. The spin quantum number indicates that the number of possible states for an electron in an orbital is a. 1. c. 3. b. 2. d. 5. ____ 39. The spin quantum number of an electron can be thought of as describing a. the direction of electron spin. b. whether the electron's charge is positive or ne ...
513 100 Note_Atom - Chemistry Silpakorn University
... than cathode ray for a given value of the field. – much smaller charge-to-mass ratio than that of cathode ray – Charge-to-mass values vary depending on the nature of the gas in the tube Particles of higher mass than electron Dr. Nattawan Worawannotai, Department of Chemistry, Faculty of Science, Sil ...
... than cathode ray for a given value of the field. – much smaller charge-to-mass ratio than that of cathode ray – Charge-to-mass values vary depending on the nature of the gas in the tube Particles of higher mass than electron Dr. Nattawan Worawannotai, Department of Chemistry, Faculty of Science, Sil ...
bYTEBoss Chapter 4 Relative atomic mass and the mole
... system which contains as many elementary entities as there are atoms in 12 g (0.012 kilograms) of carbon 12. • That number is 6.02 X1023 or ...
... system which contains as many elementary entities as there are atoms in 12 g (0.012 kilograms) of carbon 12. • That number is 6.02 X1023 or ...
Atomic Theories and Models
... 1. What were Leucippus and Democritus ideas regarding matter? 2. Describe what these philosophers thought the atom looked like? 3. How were the ideas of these two men received by Aristotle, and what was the result on the progress of atomic theory for the next couple thousand years? Alchemists: Go to ...
... 1. What were Leucippus and Democritus ideas regarding matter? 2. Describe what these philosophers thought the atom looked like? 3. How were the ideas of these two men received by Aristotle, and what was the result on the progress of atomic theory for the next couple thousand years? Alchemists: Go to ...
Hydrogen Storage in Magnesium Clusters
... effect is observed with other bulk phases of magnesium hydride.9,13,29-33 γ-MgH2 is a less stable phase than the more common β-MgH2, but the improvement in desorption temperature is lost after the first hydrogenation/dehydrogenation cycle, upon which β-MgH2 is formed. Lower desorption temperatures h ...
... effect is observed with other bulk phases of magnesium hydride.9,13,29-33 γ-MgH2 is a less stable phase than the more common β-MgH2, but the improvement in desorption temperature is lost after the first hydrogenation/dehydrogenation cycle, upon which β-MgH2 is formed. Lower desorption temperatures h ...
Summer Work
... Third Exercise: Writing the balanced ionic Equation, predict the products for the following solutions are combined. Circle the precipitate (if any), place a box around the spectator ions. a. potassium chloride(aq) + silver(I) nitrate(aq) → b. lead (II) nitrate(aq) + hydrogen chloride(aq) → c. sodium ...
... Third Exercise: Writing the balanced ionic Equation, predict the products for the following solutions are combined. Circle the precipitate (if any), place a box around the spectator ions. a. potassium chloride(aq) + silver(I) nitrate(aq) → b. lead (II) nitrate(aq) + hydrogen chloride(aq) → c. sodium ...
n=1 l=0
... Bohr’s equation also does well for heavier atoms IF they have been ionized such that only one electron remains in its orbits. For example for Helium (2 protons (Z=2) in the nucleus), 2 electrons in the orbits would make it neutral, but only if one is missing can Bohr’s equations be applied. The equa ...
... Bohr’s equation also does well for heavier atoms IF they have been ionized such that only one electron remains in its orbits. For example for Helium (2 protons (Z=2) in the nucleus), 2 electrons in the orbits would make it neutral, but only if one is missing can Bohr’s equations be applied. The equa ...
protons, neutrons, electrons
... 1. Count out 50 whole lima beans from the Ziploc bag. Place the others back in the bag. 2. Place the 50 lima beans on the platform of the balance and determine the mass to the nearest tenth of a gram. 3. Record this mass on your Data Chart. (See below) 4. Now find the average mass of these 50 lima b ...
... 1. Count out 50 whole lima beans from the Ziploc bag. Place the others back in the bag. 2. Place the 50 lima beans on the platform of the balance and determine the mass to the nearest tenth of a gram. 3. Record this mass on your Data Chart. (See below) 4. Now find the average mass of these 50 lima b ...
Chemical Equations and Reactions
... Word and Formula Equations • To complete the process of writing a correct equation, the law of conservation of mass must be taken into account. • The relative amounts of reactants and products represented in the equation must be adjusted so that the numbers and types of atoms are the same on both s ...
... Word and Formula Equations • To complete the process of writing a correct equation, the law of conservation of mass must be taken into account. • The relative amounts of reactants and products represented in the equation must be adjusted so that the numbers and types of atoms are the same on both s ...
File
... a) Identify the separation technique from the list above. b) Select the property that allows for the technique to work, from among these choices: 1. Differences in particle “attractiveness” or “electrical stickiness” of the mixture components, allowing them to be separated when solvent and some othe ...
... a) Identify the separation technique from the list above. b) Select the property that allows for the technique to work, from among these choices: 1. Differences in particle “attractiveness” or “electrical stickiness” of the mixture components, allowing them to be separated when solvent and some othe ...
Book: The Structure of Atoms
... have different properties? Why are they gases, liquids, solids, metals, nonmetals, and so on? Why do some groups of elements have similar properties and form compounds with similar formulas? The answers to these and many other fascinating questions in chemistry are supplied by our modern understandi ...
... have different properties? Why are they gases, liquids, solids, metals, nonmetals, and so on? Why do some groups of elements have similar properties and form compounds with similar formulas? The answers to these and many other fascinating questions in chemistry are supplied by our modern understandi ...
Main-group elements as transition metals
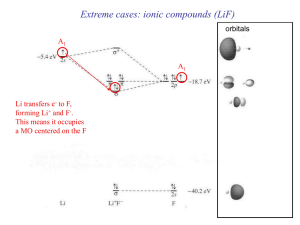
... and it is possible to write these distorted structures using a valencebond approach (Fig. 1d), analogous to Lappert’s representations of the ethylene analogues (Fig. 1b and c). In essence, the heavier alkyne analogues also contain an increasing degree of non-bonded electron localization at the heavi ...
... and it is possible to write these distorted structures using a valencebond approach (Fig. 1d), analogous to Lappert’s representations of the ethylene analogues (Fig. 1b and c). In essence, the heavier alkyne analogues also contain an increasing degree of non-bonded electron localization at the heavi ...
Writing Chemical Formulas
... Remember - subscripts of 1 are never written in a formula! Identify the oxidation number for the element making up each half (positive & negative) of the compound. Use the oxidation number (without the plus or minus) for each half as the subscript for the other half. Do not write a subscript of 1. R ...
... Remember - subscripts of 1 are never written in a formula! Identify the oxidation number for the element making up each half (positive & negative) of the compound. Use the oxidation number (without the plus or minus) for each half as the subscript for the other half. Do not write a subscript of 1. R ...
Solution
... You decide to carry out this reaction in your flask. The equilibrium constant for this reaction is K and the reaction is known to be endothermic. Assume that you start with only gas A in your flask and you let the system come to equilibrium. Now, you place your flask in the refrigerator (i.e. you su ...
... You decide to carry out this reaction in your flask. The equilibrium constant for this reaction is K and the reaction is known to be endothermic. Assume that you start with only gas A in your flask and you let the system come to equilibrium. Now, you place your flask in the refrigerator (i.e. you su ...
S8 + ___ F2 → ___ SF6 - Canvas by Instructure
... 1. The oxidation number of atoms in their elemental form is zero. 2. The oxidation state of monatomic ions is the same as the charge. 3. The oxidation state of fluorine is always -1 in its compounds. 4. The oxidation state of other halogens (Cl, Br, I) is -1 unless combined with O, F, or a more reac ...
... 1. The oxidation number of atoms in their elemental form is zero. 2. The oxidation state of monatomic ions is the same as the charge. 3. The oxidation state of fluorine is always -1 in its compounds. 4. The oxidation state of other halogens (Cl, Br, I) is -1 unless combined with O, F, or a more reac ...
AP Chemistry - cloudfront.net
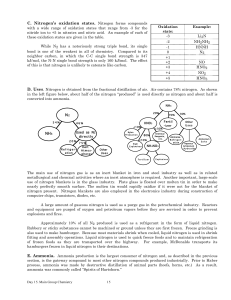
... 8.37 Which group in the periodic table has elements with high IE1 and very negative first electron affinities (EA1)? What is the charge on the ions that these atoms form? 8.59 Write the charge and full ground-state electron configuration of the monatomic ion most likely to be formed by each of the f ...
... 8.37 Which group in the periodic table has elements with high IE1 and very negative first electron affinities (EA1)? What is the charge on the ions that these atoms form? 8.59 Write the charge and full ground-state electron configuration of the monatomic ion most likely to be formed by each of the f ...
Chemistry B – Introduction to Chemical Reactions
... labs. These assessments are based on accuracy. Formative assignments will be worth 20% of your grade. Formative assignments include homework, projects and practice quizzes. Formative assignments grades are based primarily on effort. Below is the point allocation for chemistry assignments. Rememb ...
... labs. These assessments are based on accuracy. Formative assignments will be worth 20% of your grade. Formative assignments include homework, projects and practice quizzes. Formative assignments grades are based primarily on effort. Below is the point allocation for chemistry assignments. Rememb ...
answers to part a of the national high school
... chlorates are also considered to be oxidising substances because they can release oxygen, nitrogen dioxide etc. when they are heated. The fuel indicated in the triangle could be any flammable substance, not just gasoline or oil etc. It could be wood or paper or absolutely anything that can burn. A ...
... chlorates are also considered to be oxidising substances because they can release oxygen, nitrogen dioxide etc. when they are heated. The fuel indicated in the triangle could be any flammable substance, not just gasoline or oil etc. It could be wood or paper or absolutely anything that can burn. A ...
Nitrogen`s oxidation states
... which each P atom is bonded to three other P atoms. The sp3 lone pair is directed outwards from each atom. White phosphorus is extremely reactive. It catches fire immediately in air and is usually stored under water in which it is insoluble. White phosphorus must never be directly handled because it ...
... which each P atom is bonded to three other P atoms. The sp3 lone pair is directed outwards from each atom. White phosphorus is extremely reactive. It catches fire immediately in air and is usually stored under water in which it is insoluble. White phosphorus must never be directly handled because it ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.