PowerPoint Presentation - Nerve activates contraction
... Molecule—two or more atoms of the same elements combined chemically Example of a chemical reaction resulting in a molecule: H (atom) H (atom) H2 (molecule) The reactants are the atoms on the left The product is the molecule on the right represented by a molecular formula ...
... Molecule—two or more atoms of the same elements combined chemically Example of a chemical reaction resulting in a molecule: H (atom) H (atom) H2 (molecule) The reactants are the atoms on the left The product is the molecule on the right represented by a molecular formula ...
Chapter 7: The Mole and Chemical Composition
... Notice that the unit of mole is abbreviated (mol), we like to abbreviate whenever possible in chemistry, even if it is only 1 letter… You can also use this same technique to convert from moles of an element or compound to grams of that element or compound. See…we use a balance to measure out grams ...
... Notice that the unit of mole is abbreviated (mol), we like to abbreviate whenever possible in chemistry, even if it is only 1 letter… You can also use this same technique to convert from moles of an element or compound to grams of that element or compound. See…we use a balance to measure out grams ...
Chapter 4: The Structure of the Atom
... they were fairly convinced by the end of the 1800s of the following: • Cathode rays were a stream of charged particles. • The particles carried a negative charge. (The exact value of the negative charge was not known.) Because changing the metal that makes up the electrodes or varying the gas (at ve ...
... they were fairly convinced by the end of the 1800s of the following: • Cathode rays were a stream of charged particles. • The particles carried a negative charge. (The exact value of the negative charge was not known.) Because changing the metal that makes up the electrodes or varying the gas (at ve ...
Personal Tutor - Macmillan Learning
... two meters high. However, many lengths we may wish to measure are either much larger (distance from the earth to the sun) or much smaller (width of a dime or the size of an atomic nucleus) than a meter. To handle such measurements easily, common metric prefixes are used to change the size of the uni ...
... two meters high. However, many lengths we may wish to measure are either much larger (distance from the earth to the sun) or much smaller (width of a dime or the size of an atomic nucleus) than a meter. To handle such measurements easily, common metric prefixes are used to change the size of the uni ...
CHEM110P1_06_2015_Y_P1
... (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH solution and 20.00 mL aliquots of the acid solution were titrated. The titration dat ...
... (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH solution and 20.00 mL aliquots of the acid solution were titrated. The titration dat ...
Document
... Charge Distribution in a Water Molecule • There is an uneven distribution of electrons within the water molecule. – This causes the oxygen side of the molecule to have a partial negative charge (d–) and the hydrogen side to have a partial positive ...
... Charge Distribution in a Water Molecule • There is an uneven distribution of electrons within the water molecule. – This causes the oxygen side of the molecule to have a partial negative charge (d–) and the hydrogen side to have a partial positive ...
Spring 2014
... that the work of others in this class has, to the best of my knowledge, been honest as well. Signed __________________________________________________________________________ If you feel you can’t sign this, contact the instructor (e-mail or in person) (2 pts each) Multiple choice - circle the corre ...
... that the work of others in this class has, to the best of my knowledge, been honest as well. Signed __________________________________________________________________________ If you feel you can’t sign this, contact the instructor (e-mail or in person) (2 pts each) Multiple choice - circle the corre ...
Chapter 2 - Schoolwires.net
... Chemical reactions occur when atoms combine with or dissociate from other atoms Atoms are united by chemical bonds Atoms dissociate from other atoms when chemical bonds are broken ...
... Chemical reactions occur when atoms combine with or dissociate from other atoms Atoms are united by chemical bonds Atoms dissociate from other atoms when chemical bonds are broken ...
File
... us the mole ratio. • It takes 1 mole of ethanol to react with 3 moles of oxygen. This produces 2 moles of carbon dioxide and 3 moles of water. • The mole ratio will act as our conversion ...
... us the mole ratio. • It takes 1 mole of ethanol to react with 3 moles of oxygen. This produces 2 moles of carbon dioxide and 3 moles of water. • The mole ratio will act as our conversion ...
Mass Relationships in Chemical Reactions
... (vitamin C: cures/prevents scurvy). It is composed of 40.92% C, 4.58% H, and 54.50% O by mass. To determine the empirical formula, we will first assume a 100 g sample of Ascorbic Acid ...
... (vitamin C: cures/prevents scurvy). It is composed of 40.92% C, 4.58% H, and 54.50% O by mass. To determine the empirical formula, we will first assume a 100 g sample of Ascorbic Acid ...
First Semester Final Review
... percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the hydrate sample to spatter out of the crucible. b. The dehydrated sample absorbed moisture after heating. c. The amount of the hydrate sample used was too small. d. The cru ...
... percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the hydrate sample to spatter out of the crucible. b. The dehydrated sample absorbed moisture after heating. c. The amount of the hydrate sample used was too small. d. The cru ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Camp 1 - drjosephryan.com Home Page
... Reactions Between Ions – we can simplify the equation for the formation ...
... Reactions Between Ions – we can simplify the equation for the formation ...
Name __KEY____________ Per. ______ Polarity and
... (hold onto/ let go of) their electrons. For any two elements that share a chemical bond, we can calculate the difference in electronegativity by _subtracting_ (multiplying/ subtracting/ adding) their electronegativity values that we can get from a table. In our textbook there is a table on page 177. ...
... (hold onto/ let go of) their electrons. For any two elements that share a chemical bond, we can calculate the difference in electronegativity by _subtracting_ (multiplying/ subtracting/ adding) their electronegativity values that we can get from a table. In our textbook there is a table on page 177. ...
Atomic Theory PPT
... Summary Both of these samples contain the same substance. Even though there are different quantities in each tube, the ratios or proportions of the elements to one another by mass are the same. Each contains: 1.12 g chlorine 1.00 g copper This is known as The Law of Definite Proportions. End of sect ...
... Summary Both of these samples contain the same substance. Even though there are different quantities in each tube, the ratios or proportions of the elements to one another by mass are the same. Each contains: 1.12 g chlorine 1.00 g copper This is known as The Law of Definite Proportions. End of sect ...
03 Stoichiometry
... DIMENSIONAL ANALYSIS DISCLAIMER: Beginning on page 84 of the Chapter 3 text files you can find on this CD, you can find all of the remaining exercises worked out with dimensional analysis. This is most likely the way you were taught in Chemistry I. I will show you some alternatives to dimensional an ...
... DIMENSIONAL ANALYSIS DISCLAIMER: Beginning on page 84 of the Chapter 3 text files you can find on this CD, you can find all of the remaining exercises worked out with dimensional analysis. This is most likely the way you were taught in Chemistry I. I will show you some alternatives to dimensional an ...
Naming Simple Compounds
... Dalton’s Atomic Theory Gay-Lussac and Avogadro (1809—1811) Gay—Lussac Measured (under same conditions of T and P) the volumes of gases that reacted with each other. Avogadro’s Hypothesis At the same T and P, equal volumes of different gases contain the same number of particles. Volume of a gas is de ...
... Dalton’s Atomic Theory Gay-Lussac and Avogadro (1809—1811) Gay—Lussac Measured (under same conditions of T and P) the volumes of gases that reacted with each other. Avogadro’s Hypothesis At the same T and P, equal volumes of different gases contain the same number of particles. Volume of a gas is de ...
Chapter 6 Chemical reactions Classification And Mass Relationships
... get equal numbers by taking equal weights. For example since one grape weighs less than one cabbage, one pound of each will have different numbers. • The same is true for atoms or molecules of different substances. Equal numbers hydrogen and glucose molecules always have a mass ratio equal to the ra ...
... get equal numbers by taking equal weights. For example since one grape weighs less than one cabbage, one pound of each will have different numbers. • The same is true for atoms or molecules of different substances. Equal numbers hydrogen and glucose molecules always have a mass ratio equal to the ra ...
Chapter 2 - HCC Learning Web
... Atomic Number (Z) – number of protons Mass Number (A) – number of protons plus number of neutrons ...
... Atomic Number (Z) – number of protons Mass Number (A) – number of protons plus number of neutrons ...
Ch2
... Why 1/12 the mass of Carbon-12? You might think that this would work with any particular isotope (e.g. 1/7 the mass of Lithium-7). The problem is that there is a difference in the mass of the atom as a whole, and the sum of the masses of its individual protons, neutrons, and electrons. When the par ...
... Why 1/12 the mass of Carbon-12? You might think that this would work with any particular isotope (e.g. 1/7 the mass of Lithium-7). The problem is that there is a difference in the mass of the atom as a whole, and the sum of the masses of its individual protons, neutrons, and electrons. When the par ...
Ch:3
... Niels Bohr proposed in 1914 a model of the hydrogen atom as a nucleus with an electron circling around it. ...
... Niels Bohr proposed in 1914 a model of the hydrogen atom as a nucleus with an electron circling around it. ...
2. Chapter 2
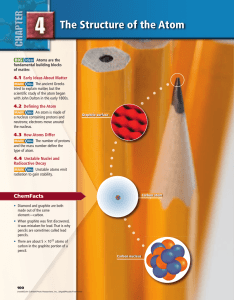
... You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken down further is that it is already very simple: each element is made of only one kind of atom. Elements can be found in your pencils, your coins, ...
... You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken down further is that it is already very simple: each element is made of only one kind of atom. Elements can be found in your pencils, your coins, ...
Atomic mass
... Why 1/12 the mass of Carbon-12? You might think that this would work with any particular isotope (e.g. 1/7 the mass of Lithium-7). The problem is that there is a difference in the mass of the atom as a whole, and the sum of the masses of its individual protons, neutrons, and electrons. When the par ...
... Why 1/12 the mass of Carbon-12? You might think that this would work with any particular isotope (e.g. 1/7 the mass of Lithium-7). The problem is that there is a difference in the mass of the atom as a whole, and the sum of the masses of its individual protons, neutrons, and electrons. When the par ...
Chapter 3 Notes
... 4. Check your work by counting atoms of each element , they should be the same on both sides of the equation. 5. If you cannot balance an equation, you have probably written a formula incorrectly, Check and try again. Hints for balancing and writing chemical equations: * Remember the seven elements ...
... 4. Check your work by counting atoms of each element , they should be the same on both sides of the equation. 5. If you cannot balance an equation, you have probably written a formula incorrectly, Check and try again. Hints for balancing and writing chemical equations: * Remember the seven elements ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.