Grade 8 th Science Curriculum Scope and Sequence
... Use evidence from investigations to describe the effect that adding heat energy to different types of matter has on the rate at which the matter changes from one state to another. b. Based on data from investigations describe the effect that removing heat energy from different types of matter has on ...
... Use evidence from investigations to describe the effect that adding heat energy to different types of matter has on the rate at which the matter changes from one state to another. b. Based on data from investigations describe the effect that removing heat energy from different types of matter has on ...
Gmelin Tips and Reminders
... Structure Searching • Not all substances have a structure associated. Only compounds or compound parts, which are formed by discrete molecules or ions, have a structure. Compounds with a solid-state structure will not have a structure in the Gmelin database. If your search by structure brings up 0 h ...
... Structure Searching • Not all substances have a structure associated. Only compounds or compound parts, which are formed by discrete molecules or ions, have a structure. Compounds with a solid-state structure will not have a structure in the Gmelin database. If your search by structure brings up 0 h ...
Chapter 2: Atoms, Ions, and the Periodic Table
... E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theory? A) All matter is composed of small indivisible particles called atoms. B) All atoms of a given ele ...
... E) When water is broken down into its elements by electrolysis, elemental oxygen and hydrogen are formed in an 8 to 1 mass ratio. Ans: C 8. Which of the following is not part of Dalton's atomic theory? A) All matter is composed of small indivisible particles called atoms. B) All atoms of a given ele ...
Chapter 2: Atoms, Ions, and the Periodic Table
... D) Dalton's atomic theory says that a chemical reaction is a rearrangement of atoms into one or more different chemical substances. E) Joseph Proust's findings regarding the reactions between metals and oxygen led to the law of definite proportions. Ans: A 10. Dalton's atomic theory consisted of all ...
... D) Dalton's atomic theory says that a chemical reaction is a rearrangement of atoms into one or more different chemical substances. E) Joseph Proust's findings regarding the reactions between metals and oxygen led to the law of definite proportions. Ans: A 10. Dalton's atomic theory consisted of all ...
Physical Science Chapter 7 Chemical Reactions Section 7.1
... As calcium atoms lose electrons during the synthesis of calcium oxide, the oxygen atoms gain electrons. As each neutral oxygen atom gains two electrons, it becomes an ion with a charge of 2–. ____________________ ___________________________________________________________________________. ...
... As calcium atoms lose electrons during the synthesis of calcium oxide, the oxygen atoms gain electrons. As each neutral oxygen atom gains two electrons, it becomes an ion with a charge of 2–. ____________________ ___________________________________________________________________________. ...
Document
... Proton number never changes for any given element. For example, oxygen has an atomic number of 8 indicating that oxygen always has 8 protons. ...
... Proton number never changes for any given element. For example, oxygen has an atomic number of 8 indicating that oxygen always has 8 protons. ...
mystery elements
... hypothesized about the atom, but were unable to run experiments on it. What does the law of conservation of mass state? ...
... hypothesized about the atom, but were unable to run experiments on it. What does the law of conservation of mass state? ...
Teacher Background - Online Learning Exchange
... mole is equivalent to 6.02 1023 particles of a substance.) Ask: How can you determine the number of moles of a substance in a chemical equation? (The number of moles is represented by the substance’s coefficient.) Ask: What is molar mass? (Molar mass is the mass of one mole of a substance.) ...
... mole is equivalent to 6.02 1023 particles of a substance.) Ask: How can you determine the number of moles of a substance in a chemical equation? (The number of moles is represented by the substance’s coefficient.) Ask: What is molar mass? (Molar mass is the mass of one mole of a substance.) ...
Sorenson, Ch.1
... shells of an atom, where they are most "tightly bound" to the nucleus. For example, in carbon, which has six electrons, two electrons (the maximum number allowed) occupy the K shell, and the four remaining electrons are found in the L shell. Electrons can be moved to higher shells or completely remo ...
... shells of an atom, where they are most "tightly bound" to the nucleus. For example, in carbon, which has six electrons, two electrons (the maximum number allowed) occupy the K shell, and the four remaining electrons are found in the L shell. Electrons can be moved to higher shells or completely remo ...
chemistry
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... ai)................................ so it is easy to draw out into wires. ...
... ai)................................ so it is easy to draw out into wires. ...
Masses of Atoms
... 80% of Boron in nature have 5 protons, 6 neutrons ~ 11 amu 20% of Boron in nature have 5 protons, 5 neutrons ~ 10 amu (.8 • 11 amu) + (.2 • 10 amu) = 8.8 amu + 2 amu = 10.8 amu There are a few extra isotopes out there that we did not include. ...
... 80% of Boron in nature have 5 protons, 6 neutrons ~ 11 amu 20% of Boron in nature have 5 protons, 5 neutrons ~ 10 amu (.8 • 11 amu) + (.2 • 10 amu) = 8.8 amu + 2 amu = 10.8 amu There are a few extra isotopes out there that we did not include. ...
Physical Earth Daily Learning Guide DRAFT - Burlington
... 9-11 PS2A Atomic structure and subatomic particles. 9-11 PS2B Atoms structure is related to physical and chemical properties. 9-11 PS2C Periodic trends are related to atomic structure. 9-11 PS2D The formation of ions and ionic compounds. 9-11 PS2E Covalent bonding and molecular substances. 9-11 PS2F ...
... 9-11 PS2A Atomic structure and subatomic particles. 9-11 PS2B Atoms structure is related to physical and chemical properties. 9-11 PS2C Periodic trends are related to atomic structure. 9-11 PS2D The formation of ions and ionic compounds. 9-11 PS2E Covalent bonding and molecular substances. 9-11 PS2F ...
Atomic Electron Configurations and Chapter 8 Chemical Periodicity
... B. The nucleus becomes bigger in size as it has more protons and neutrons C Down the group C. group, new shells (i (i.e. e n is increased by 1) are added; each new shell is further and further away from the nucleus D. The nucleus expands and the shells (filled with electrons) expands ...
... B. The nucleus becomes bigger in size as it has more protons and neutrons C Down the group C. group, new shells (i (i.e. e n is increased by 1) are added; each new shell is further and further away from the nucleus D. The nucleus expands and the shells (filled with electrons) expands ...
Document
... • Empirical formulas give only the smallest whole number ratio of atoms in a compound. • Molecular formulas give the actual number of each type of atom within a compound. • Compounds with the same empirical formula but different molecular formulas can have very different properties. • For example, C ...
... • Empirical formulas give only the smallest whole number ratio of atoms in a compound. • Molecular formulas give the actual number of each type of atom within a compound. • Compounds with the same empirical formula but different molecular formulas can have very different properties. • For example, C ...
Chapter 3 – Atomic Structure and Properties
... Rather, they are inferred from the distances between atoms in molecules, which can be readily determined with several techniques. However, a discussion of how atomic radii are defined requires an understanding of chemical bonding and the solid state, so a detailed discussion of the different types o ...
... Rather, they are inferred from the distances between atoms in molecules, which can be readily determined with several techniques. However, a discussion of how atomic radii are defined requires an understanding of chemical bonding and the solid state, so a detailed discussion of the different types o ...
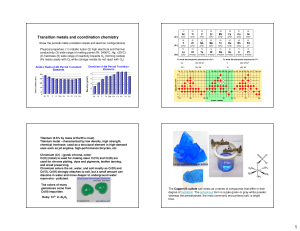
Transition metals and coordination chemistry
... degree of hydration. The anhydrous form is a pale green or gray-white powder, whereas the pentahydrate, the most commonly encountered salt, is bright blue. ...
... degree of hydration. The anhydrous form is a pale green or gray-white powder, whereas the pentahydrate, the most commonly encountered salt, is bright blue. ...
PDF | 715.3KB
... 88. An atom forms an ion which combines with the aluminum ion in a 3:1 ratio. Which group must this element belong to on the periodic table? 89. Write the correct shorthand electron configuration for magnesium. 90. Write the correct shorthand electron configuration for O291. An element has an electr ...
... 88. An atom forms an ion which combines with the aluminum ion in a 3:1 ratio. Which group must this element belong to on the periodic table? 89. Write the correct shorthand electron configuration for magnesium. 90. Write the correct shorthand electron configuration for O291. An element has an electr ...
The structure and mass of atoms - Brentwood Ursuline Convent
... Figure 1 Close-up of an atom. Note that the sizes of the protons, neutrons and electrons are not to scale. ...
... Figure 1 Close-up of an atom. Note that the sizes of the protons, neutrons and electrons are not to scale. ...
Examples
... The same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) – molar mass is just a much broader term than these other specific masses ...
... The same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) – molar mass is just a much broader term than these other specific masses ...
Moles - tamchemistryhart
... Molar mass = mass, in grams, of 1 mole of a substance (6.02 x 1023 particles) expressed in g/mol Molar mass is numerically equal to average atomic mass in amus (atomic mass units). ...
... Molar mass = mass, in grams, of 1 mole of a substance (6.02 x 1023 particles) expressed in g/mol Molar mass is numerically equal to average atomic mass in amus (atomic mass units). ...
Materials Required
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.