1. Atoms that have eight valence electrons would tend to A) be very
... be very reactive. be inert. form positive ions. form negative ions. ...
... be very reactive. be inert. form positive ions. form negative ions. ...
Periodic Table
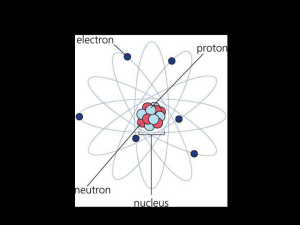
... elements, and periodic tables. First, the students will learn about the components of an atom. They will know that atoms are made of 3 types of particles electrons, protons, and neutrons. These particles have different properties. The students will build their own atom by using different colored ...
... elements, and periodic tables. First, the students will learn about the components of an atom. They will know that atoms are made of 3 types of particles electrons, protons, and neutrons. These particles have different properties. The students will build their own atom by using different colored ...
Chapter #2-Newest CPub
... same temperature and pressure) are in the ratio of simple integers. Moreover, the ratio of the volume of each product gas to the volume of either reacting gas is the ratio of simple integers. Avogadro’s Hypothesis ...
... same temperature and pressure) are in the ratio of simple integers. Moreover, the ratio of the volume of each product gas to the volume of either reacting gas is the ratio of simple integers. Avogadro’s Hypothesis ...
Atoms, Elements, and Compounds
... An element is a pure substance that cannot be broken down into other substances by physical or chemical means. Elements are made of only one type of atom. There are 118 known elements, 94 occur naturally. ...
... An element is a pure substance that cannot be broken down into other substances by physical or chemical means. Elements are made of only one type of atom. There are 118 known elements, 94 occur naturally. ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
Document
... of atoms of different elements in fixed ratios Are represented by formulas Molecular formula-symbols and subscripts are used to represent elements and their ratios in each molecule of a compound Structural formula-shows how the atoms are connected in a molecule using symbols to represent atoms and ...
... of atoms of different elements in fixed ratios Are represented by formulas Molecular formula-symbols and subscripts are used to represent elements and their ratios in each molecule of a compound Structural formula-shows how the atoms are connected in a molecule using symbols to represent atoms and ...
What are Valence Electrons
... Number of Valence Electrons determined by place in the Periodic Table • For elements in groups 1-2 and 13-18, the rons number of valence elect_________ is easy to tell directly from the periodic table. • For elements in group 3-12, determining the number of valence electrons is more ...
... Number of Valence Electrons determined by place in the Periodic Table • For elements in groups 1-2 and 13-18, the rons number of valence elect_________ is easy to tell directly from the periodic table. • For elements in group 3-12, determining the number of valence electrons is more ...
s8.1toatomicmass
... 1. Discovered alpha (), beta (), and gamma () particles. 2. Correctly interpreted the nature of radioactivity. 3. Discovered the true nature of the alpha particles. 4. Discovered the atomic nucleus. - Most alpha particles shot through a thin gold foil, but a few alpha particles did not penetrate ...
... 1. Discovered alpha (), beta (), and gamma () particles. 2. Correctly interpreted the nature of radioactivity. 3. Discovered the true nature of the alpha particles. 4. Discovered the atomic nucleus. - Most alpha particles shot through a thin gold foil, but a few alpha particles did not penetrate ...
Chapter 10 The Periodic Law
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
- Science
... In the case of atoms, scientists use large models to explain something that is very small Models of the atom were used to explain data or facts that were gathered experimentally. So, these models are also theories ...
... In the case of atoms, scientists use large models to explain something that is very small Models of the atom were used to explain data or facts that were gathered experimentally. So, these models are also theories ...
Helpful Science Notes Chapter 4.2 The Structure of an Atom
... Negatively charged subatomic particle that is found in the space outside the nucleus. ...
... Negatively charged subatomic particle that is found in the space outside the nucleus. ...
Final Exam Practice Problems Set 2
... 3) Atoms of an element are not changed into different types of atoms by chemical reactions; atoms are neither created nor destroyed in chemical reactions. 4) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. 5) E ...
... 3) Atoms of an element are not changed into different types of atoms by chemical reactions; atoms are neither created nor destroyed in chemical reactions. 4) Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. 5) E ...
Atoms 1 ppt
... An “s” orbital is shaped like a sphere and can hold a maximum of 2 electrons. Each “p” orbital is shaped like a bar bell. There are 3 different types that can each hold 2 electrons. The “p” orbital, therefore, can hold up to 6 electrons. “d” and “f” orbitals are more complex. There are 5 types of “d ...
... An “s” orbital is shaped like a sphere and can hold a maximum of 2 electrons. Each “p” orbital is shaped like a bar bell. There are 3 different types that can each hold 2 electrons. The “p” orbital, therefore, can hold up to 6 electrons. “d” and “f” orbitals are more complex. There are 5 types of “d ...
Atomic Structure Test Review Answer Key - Unit 1
... have the same 4 quantum numbers.) q. Heisenberg uncertainty principle- you cannot know the location and velocity of an electron at same time. r. atomic emission spectrumfrequencies of light emitted by an element. s. Frequency- number of wave cycles per second (s-1) t. Wavelength- distance from equiv ...
... have the same 4 quantum numbers.) q. Heisenberg uncertainty principle- you cannot know the location and velocity of an electron at same time. r. atomic emission spectrumfrequencies of light emitted by an element. s. Frequency- number of wave cycles per second (s-1) t. Wavelength- distance from equiv ...
The atomic number tells how many protons Protons make an atom
... many protons AND neutrons there are in the nucleus The number of neutrons do NOT make a difference in the identity of an atom Atoms of the same element will ALWAYS have the same # of protons, but MAY have different neutrons ...
... many protons AND neutrons there are in the nucleus The number of neutrons do NOT make a difference in the identity of an atom Atoms of the same element will ALWAYS have the same # of protons, but MAY have different neutrons ...
Chapter 2 Practice Questions
... C) All atoms of a given element are identical. D) Atoms are indivisible in chemical reactions. E) All of these statements are true according to modern atomic theory. 4. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound al ...
... C) All atoms of a given element are identical. D) Atoms are indivisible in chemical reactions. E) All of these statements are true according to modern atomic theory. 4. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound al ...
the_atom_ppt
... Early Models of the Atom Dalton • All elements are composed of indivisible particles. • Atoms of the same element are the same • Atoms of different elements are different. • Compounds consisted of atoms of different elements combined together ...
... Early Models of the Atom Dalton • All elements are composed of indivisible particles. • Atoms of the same element are the same • Atoms of different elements are different. • Compounds consisted of atoms of different elements combined together ...
- gst boces
... *<7 acidic (H+ > OH-), farther from neutral = more acidic *>7 basic (OH- . H+), farther from neutral = more basic *each move a 10x change in H+ concentration (1 is 10x stronger than 2, 1 is 100x stronger than 3) 145. All organic compounds contain C, carbon *and (usually) H, hydrogen 146. Carbon ALWA ...
... *<7 acidic (H+ > OH-), farther from neutral = more acidic *>7 basic (OH- . H+), farther from neutral = more basic *each move a 10x change in H+ concentration (1 is 10x stronger than 2, 1 is 100x stronger than 3) 145. All organic compounds contain C, carbon *and (usually) H, hydrogen 146. Carbon ALWA ...
4.1 Studying Atoms
... The alpha particles whose paths were deflected must have come close to another charged object. The closer they came, the greater the deflection. However, many alpha particles passed through the gold without being deflected. These particles did not pass close to a charged object. ...
... The alpha particles whose paths were deflected must have come close to another charged object. The closer they came, the greater the deflection. However, many alpha particles passed through the gold without being deflected. These particles did not pass close to a charged object. ...
CH 2 development of atomic theory
... observing a knife thrower at a circus. The knife thrower tries to outline his assistant with knifes. The size and shape of the assistant can be determined from the outline. The experiment had some surprising results. Most of the particles passed straight through but some were deflected, a few right ...
... observing a knife thrower at a circus. The knife thrower tries to outline his assistant with knifes. The size and shape of the assistant can be determined from the outline. The experiment had some surprising results. Most of the particles passed straight through but some were deflected, a few right ...
Chemistry: The Nature of Matter
... o 2nd shell has a little more energy and holds 8 electrons o 3rd shell has even more energy, etc. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Electron config ...
... o 2nd shell has a little more energy and holds 8 electrons o 3rd shell has even more energy, etc. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Electron config ...
Atomic Weights Average Atomic Masses
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.