History of the Atom
... History of the Atom • We know today that all matter is composed of atoms. • Atoms: basic unit of matter. Atoms are the smallest particle of an element that retains its identity. How did scientists figure out the structure of the atom? Can you just "look" at it? ...
... History of the Atom • We know today that all matter is composed of atoms. • Atoms: basic unit of matter. Atoms are the smallest particle of an element that retains its identity. How did scientists figure out the structure of the atom? Can you just "look" at it? ...
Chapter 2: Atoms Molecules and Ions
... Greeks were the first to attempt to explain the nature of matter. They relied on logic & reason to explain the natural world. i) Aristotle & Plato believed that there were four elements (air, water, Earth, & fire). ii) Leucippus & Democritus proposed that elements are composed of tiny particles that ...
... Greeks were the first to attempt to explain the nature of matter. They relied on logic & reason to explain the natural world. i) Aristotle & Plato believed that there were four elements (air, water, Earth, & fire). ii) Leucippus & Democritus proposed that elements are composed of tiny particles that ...
Chapter 1 Chemistry: the study of the composition of matter and the
... Conversion questions. For example, how many grams in 27.4 kilograms? Significant figures a. Count them, round to a specific significant figure, add/subtract/multiply/divide Write in scientific notation Is it accurate? Precise? What is the percent error? Be able to solve for the missing value in a de ...
... Conversion questions. For example, how many grams in 27.4 kilograms? Significant figures a. Count them, round to a specific significant figure, add/subtract/multiply/divide Write in scientific notation Is it accurate? Precise? What is the percent error? Be able to solve for the missing value in a de ...
Presentation
... They are the smallest particle of a substance that still retains the properties of that substance and is composed of 2 or more atoms. ...
... They are the smallest particle of a substance that still retains the properties of that substance and is composed of 2 or more atoms. ...
Chemistry 11 – Course Outcomes
... Use electron affinity to explain formation of ions and explain how the formation of ionic compounds occurs State octet rule Give an electron dot diagram (Lewis dot diagram) of atoms forming ions and ionic bonding (3 equations) Use model for the structure of an ionic compound to explain its propertie ...
... Use electron affinity to explain formation of ions and explain how the formation of ionic compounds occurs State octet rule Give an electron dot diagram (Lewis dot diagram) of atoms forming ions and ionic bonding (3 equations) Use model for the structure of an ionic compound to explain its propertie ...
02_Lecture_Presentation
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
... Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be appro ...
High School Chemistry
... b. Using the periodic table, predict the charge an atom will acquire when it forms an ion by gaining or losing electrons. c. Compare covalent and ionic bonds with respect to electron behavior and relative bond strengths. d. Diagram a model of a metallic bond and explain how it differs from ionic an ...
... b. Using the periodic table, predict the charge an atom will acquire when it forms an ion by gaining or losing electrons. c. Compare covalent and ionic bonds with respect to electron behavior and relative bond strengths. d. Diagram a model of a metallic bond and explain how it differs from ionic an ...
Subject - Currituck County Schools
... Structure of Matter Illustrate how observations and conclusions from experimentation changed atomic theory over time. Explain Dalton’s atomic theory, which states the following: o Chemical elements are made up of atoms. o The atoms of an element are identical in their masses. (Be sure students under ...
... Structure of Matter Illustrate how observations and conclusions from experimentation changed atomic theory over time. Explain Dalton’s atomic theory, which states the following: o Chemical elements are made up of atoms. o The atoms of an element are identical in their masses. (Be sure students under ...
Elements and Atoms
... The reason different elements have different properties is because the atoms that make up different elements have different numbers of tiny particles that make them up. The particles that make up atoms are called subatomic particles. There are three types of subatomic particles: 1. protons (positive ...
... The reason different elements have different properties is because the atoms that make up different elements have different numbers of tiny particles that make them up. The particles that make up atoms are called subatomic particles. There are three types of subatomic particles: 1. protons (positive ...
Dalton`s Atomic Theory
... You probably know what this sketch represents. It’s a model of an atom, one of the miniscule particles that make up all matter. The idea that matter consists of extremely tiny particles called atoms was first introduced about 2500 years ago by a Greek philosopher named Democritus. However, other phi ...
... You probably know what this sketch represents. It’s a model of an atom, one of the miniscule particles that make up all matter. The idea that matter consists of extremely tiny particles called atoms was first introduced about 2500 years ago by a Greek philosopher named Democritus. However, other phi ...
Chapter 3 The Atom
... so vigorously recycled at death that a significant number of our atoms – up to a billion for each of us, probably once belonged to Shakespeare. A billion more each came from Buddha and Genghis Khan and Beethoven and …” Bill Bryson ...
... so vigorously recycled at death that a significant number of our atoms – up to a billion for each of us, probably once belonged to Shakespeare. A billion more each came from Buddha and Genghis Khan and Beethoven and …” Bill Bryson ...
Chapter 2 Atoms, Molecules and Ions
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. ...
... 4. Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms. ...
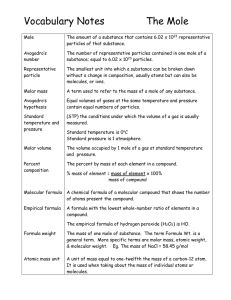
Vocabulary Notes
... The number of representative particles contained in one mole of a substance; equal to 6.02 x 1023 particles. ...
... The number of representative particles contained in one mole of a substance; equal to 6.02 x 1023 particles. ...
Grade 9 Science Unit 1 Review.notebook
... Conductivity Boiling/melting point Texture Magnetism Density ...
... Conductivity Boiling/melting point Texture Magnetism Density ...
Final Exam Class Review - Mrs. Kittrell`s Science Classes
... of the most likely locations for electrons in an atom. ...
... of the most likely locations for electrons in an atom. ...
Chapter 8 & 9 PowerPoint
... Three types of bonding • Metallic bonding – results from the attraction between metal atoms and the surrounding sea of electrons • Ionic bonding – results from the electrical attraction between positive and negative ions. • Covalent bonding – results from the sharing of electron pairs between two a ...
... Three types of bonding • Metallic bonding – results from the attraction between metal atoms and the surrounding sea of electrons • Ionic bonding – results from the electrical attraction between positive and negative ions. • Covalent bonding – results from the sharing of electron pairs between two a ...
L41 - Atomic Structure
... n (principal quantum number) = 1,2,3,… RH (Rydberg constant) = 2.18 x 10-18J ...
... n (principal quantum number) = 1,2,3,… RH (Rydberg constant) = 2.18 x 10-18J ...
chpt 11 and 12 notes with answers
... Small hard particles that make up stuff (matter) Countered by Aristotle, stated never end up with something that cannot be divided ...
... Small hard particles that make up stuff (matter) Countered by Aristotle, stated never end up with something that cannot be divided ...
atoms of different elements differ in size, mass
... Most of the particles passed right through A few particles were deflected VERY FEW were greatly deflected ...
... Most of the particles passed right through A few particles were deflected VERY FEW were greatly deflected ...
Constructing an Atom We`re going sub-atomic!
... depends if an Atom is negatively charged or positively charge. – So if it has more protons then electrons then it will be _________________? – If it has more Electrons then Proton then it will be ...
... depends if an Atom is negatively charged or positively charge. – So if it has more protons then electrons then it will be _________________? – If it has more Electrons then Proton then it will be ...
Lab Science 9 Pacing Guide
... 4. Show that when elements are listed in order according to the number of protons (called the atomic number), the repeating patterns of physical and chemical properties identify families of elements. Recognize that the periodic table was formed as a result of the repeating pattern of electron config ...
... 4. Show that when elements are listed in order according to the number of protons (called the atomic number), the repeating patterns of physical and chemical properties identify families of elements. Recognize that the periodic table was formed as a result of the repeating pattern of electron config ...
bluevale collegiate institute
... A) A positively charged nucleus, consisting of protons and neutrons, orbited by electrons. B) Electrons and protons within the nucleus, orbited by neutrons. C) A dense, positively charged nucleus, orbited by protons and electrons. D) Negatively charged protons and neutrons in the nucleus, orbited by ...
... A) A positively charged nucleus, consisting of protons and neutrons, orbited by electrons. B) Electrons and protons within the nucleus, orbited by neutrons. C) A dense, positively charged nucleus, orbited by protons and electrons. D) Negatively charged protons and neutrons in the nucleus, orbited by ...
Early Greek Philosophers determined that atoms are the building
... John Dalton’s theory of the atom started out as a solid sphere with no charges. Then J.J. Thomson figured out there were positive and negative charges in an atom. Rutherford determined that the positive charges (protons) were located in the center of the atom and the negative charges (electron ...
... John Dalton’s theory of the atom started out as a solid sphere with no charges. Then J.J. Thomson figured out there were positive and negative charges in an atom. Rutherford determined that the positive charges (protons) were located in the center of the atom and the negative charges (electron ...
VOCABULARY name, date, hour: Fill in the number of each term
... ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons __ ...
... ___ stable, orbiting particle of an atom with a negative charge ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons __ ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.