Chemistry B11 Chapter 4 Chemical reactions
... Chemical Equation: we represent a chemical reaction in the form of a chemical equation, using chemical formulas for the reactants and products, and an arrow to indicate the direction in which the reaction proceeds. Note: It is important to show the state of each reactant and product in a chemical eq ...
... Chemical Equation: we represent a chemical reaction in the form of a chemical equation, using chemical formulas for the reactants and products, and an arrow to indicate the direction in which the reaction proceeds. Note: It is important to show the state of each reactant and product in a chemical eq ...
Unit 03 Packet - Whitwell High School
... In Bohr’s model of the atom, electrons are in certain ___________ levels, with the levels closest to the nucleus of __________ energy than those farther from the nucleus. In the _____________ state of the atom, the electrons are in the lowest _____________ level possible. When an atom absorbs energy ...
... In Bohr’s model of the atom, electrons are in certain ___________ levels, with the levels closest to the nucleus of __________ energy than those farther from the nucleus. In the _____________ state of the atom, the electrons are in the lowest _____________ level possible. When an atom absorbs energy ...
Periodic Table - personals.okan.edu.tr
... While F-, O= can only (–) charged ions(Anions) , the others can have –/+ ions (Anion/Cation) • -They build up covalent bonds among each other, and form ionic bonds with metals and metalloids • -They have low melting and boiling points, most of them are present at gas state at normal conditions. Br2 ...
... While F-, O= can only (–) charged ions(Anions) , the others can have –/+ ions (Anion/Cation) • -They build up covalent bonds among each other, and form ionic bonds with metals and metalloids • -They have low melting and boiling points, most of them are present at gas state at normal conditions. Br2 ...
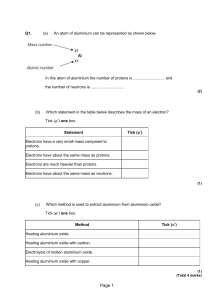
electrons = # protons
... This energy is seen as light. While the light appears as one color, it is actually composed of many different wavelengths, each of which is seen as a different line when viewed through an instrument called a spectroscope. ...
... This energy is seen as light. While the light appears as one color, it is actually composed of many different wavelengths, each of which is seen as a different line when viewed through an instrument called a spectroscope. ...
Are you ready for S279?
... chemical reactions. Atoms chemically bond with other atoms in order to achieve the stable electronic configuration of a noble gas. This can be achieved by either: (i) transferring electrons (ionic bonding) to form positive or negative ions, or (ii) sharing pairs of electrons (covalent bonding) to fo ...
... chemical reactions. Atoms chemically bond with other atoms in order to achieve the stable electronic configuration of a noble gas. This can be achieved by either: (i) transferring electrons (ionic bonding) to form positive or negative ions, or (ii) sharing pairs of electrons (covalent bonding) to fo ...
The Evolution of the Atomic Model
... Based on Balmer’s emission line studies of the hydrogen atom and Planck’s work, Bohr proposed… a planetary model in which e- could only occupy certain energy levels. energy levels differed in a step like manner. electrons can occupy different energy levels by absorbing or emitting energy. ...
... Based on Balmer’s emission line studies of the hydrogen atom and Planck’s work, Bohr proposed… a planetary model in which e- could only occupy certain energy levels. energy levels differed in a step like manner. electrons can occupy different energy levels by absorbing or emitting energy. ...
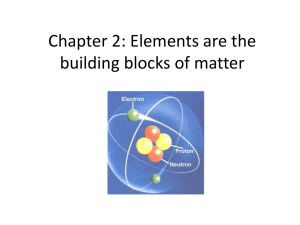
Chapter 2: Elements are the building blocks of matter
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
Chapter 4.1 Slides
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
Atomic Theory - Honors Chemistry
... Atomic mass = # of protons and neutrons each has about the same mass which is designated as an atomic mass unit (1 amu) Determination of amu one element chosen as a standard – Carbon 12 6 protons, 6 neutrons = 12 amu therefore 1 amu = 1/12th Carbon atom ...
... Atomic mass = # of protons and neutrons each has about the same mass which is designated as an atomic mass unit (1 amu) Determination of amu one element chosen as a standard – Carbon 12 6 protons, 6 neutrons = 12 amu therefore 1 amu = 1/12th Carbon atom ...
Picture Match Words Fusion Density Isotope Neutron Atomic
... b. Round 2: Each team gets a set of 4 key vocab words and blank sentence strip. The teams are asked to develop sentences using the key words. Each scientifically (1pt) + grammatically correct (1pt) sentence gets the team 2 points. ...
... b. Round 2: Each team gets a set of 4 key vocab words and blank sentence strip. The teams are asked to develop sentences using the key words. Each scientifically (1pt) + grammatically correct (1pt) sentence gets the team 2 points. ...
Chemistry Mid-Term Review: 2015-2016
... 5. What distinguishes the atoms of one element from the atoms of another? 6. What equation tells you how to calculate the number of neutrons in an atom? 7. What is the charge- positive or negative, of the nucleus of every atom? 8. Why is an atom electrically neutral? 9. What does the atomic number o ...
... 5. What distinguishes the atoms of one element from the atoms of another? 6. What equation tells you how to calculate the number of neutrons in an atom? 7. What is the charge- positive or negative, of the nucleus of every atom? 8. Why is an atom electrically neutral? 9. What does the atomic number o ...
Alcohol responsive 2D coordination network of 3
... One of the most important objectives in the field of coordination networks or metal organic frameworks is the engineering of porous structures with a well defined chemical environment [1–2]. This is however very difficult to achieve in 3D structures due to catenation and interpenetration. Thus in re ...
... One of the most important objectives in the field of coordination networks or metal organic frameworks is the engineering of porous structures with a well defined chemical environment [1–2]. This is however very difficult to achieve in 3D structures due to catenation and interpenetration. Thus in re ...
chapt 4 early atomic theory
... of one element are the same. Atoms of the same element can have different numbers of neutrons and therefore have different mass numbers and different masses. The atoms of the same element that differ in the number of neutrons are called isotopes of that ...
... of one element are the same. Atoms of the same element can have different numbers of neutrons and therefore have different mass numbers and different masses. The atoms of the same element that differ in the number of neutrons are called isotopes of that ...
PRACTICE PROBLEMS EXAM 1,2 and 3 1311
... C) +3 D) -5 E) -6 30) Horizontal rows of the periodic table are known as __________. A) periods B) groups C) metalloids D) metals E) nonmetals 31) Elements in Group 7A are known as the __________. A) chalcogens B) alkali metals C) alkaline earth metals D) halogens E) noble gases 32) When a metal and ...
... C) +3 D) -5 E) -6 30) Horizontal rows of the periodic table are known as __________. A) periods B) groups C) metalloids D) metals E) nonmetals 31) Elements in Group 7A are known as the __________. A) chalcogens B) alkali metals C) alkaline earth metals D) halogens E) noble gases 32) When a metal and ...
AP Chemistry Name: Ch.1 – Matter and Measurement Date: Period:
... 2.4 The Discovery of the Electron 1. To illustrate Robert Millikan’s determination of the charge on an electron, suppose that you were given the task of determining the mass of a single jelly bean given the following experimental data. Various scoops of jelly beans were weighed and the following mas ...
... 2.4 The Discovery of the Electron 1. To illustrate Robert Millikan’s determination of the charge on an electron, suppose that you were given the task of determining the mass of a single jelly bean given the following experimental data. Various scoops of jelly beans were weighed and the following mas ...
Summer Assignment Packet
... 2.4 The Discovery of the Electron 1. To illustrate Robert Millikan’s determination of the charge on an electron, suppose that you were given the task of determining the mass of a single jelly bean given the following experimental data. Various scoops of jelly beans were weighed and the following mas ...
... 2.4 The Discovery of the Electron 1. To illustrate Robert Millikan’s determination of the charge on an electron, suppose that you were given the task of determining the mass of a single jelly bean given the following experimental data. Various scoops of jelly beans were weighed and the following mas ...
File - Mrs. Hille`s FunZone
... Proposed that all matter acts like waves Developed the deBroglie’s hypothesis. ...
... Proposed that all matter acts like waves Developed the deBroglie’s hypothesis. ...
File
... _______3. Substance in which the atoms exist in a fixed ratio _______4. A reaction in which the composition of a substance is changed _______5. An element containing two identical atoms _______6. A change that does not alter the chemical properties of a substance _______7. Type of matter that includ ...
... _______3. Substance in which the atoms exist in a fixed ratio _______4. A reaction in which the composition of a substance is changed _______5. An element containing two identical atoms _______6. A change that does not alter the chemical properties of a substance _______7. Type of matter that includ ...
Chemistry B1A - Bakersfield College
... You create a column of the liquids in a glass cylinder. Draw a sketch and indicate which liquid is at which level in the column. Then explain what would happen if you did the following: a. First you drop a plastic bead that has a density of 0.24 g/cm3 into the column. b. You drop a bead in that make ...
... You create a column of the liquids in a glass cylinder. Draw a sketch and indicate which liquid is at which level in the column. Then explain what would happen if you did the following: a. First you drop a plastic bead that has a density of 0.24 g/cm3 into the column. b. You drop a bead in that make ...
Fundamentals of Chemistry
... The diameter of the atom is determined by the range of the electrons in their travels around the nucleus and is approximately 10-8 cm. The diameter of the nucleus is roughly 10,000 times smaller, approximately 10-13 to 10-12 cm. Because the nucleus is composed of neutrons and protons that are about ...
... The diameter of the atom is determined by the range of the electrons in their travels around the nucleus and is approximately 10-8 cm. The diameter of the nucleus is roughly 10,000 times smaller, approximately 10-13 to 10-12 cm. Because the nucleus is composed of neutrons and protons that are about ...
Chem 1100 Chapter Three Study Guide Outline I. Molar Mass and
... 15. Molecules of RNA contain the sugar D-ribose. This sugar is 40.00% C, 6.71% H and the rest oxygen by weight. What is the empirical formula for D-ribose? a. CH2O b. CH3O c. CH3O2 d. none of the above 16. A 53.00 g sample of strontium reacts with an excess of nitrogen to form strontium nitride. Wha ...
... 15. Molecules of RNA contain the sugar D-ribose. This sugar is 40.00% C, 6.71% H and the rest oxygen by weight. What is the empirical formula for D-ribose? a. CH2O b. CH3O c. CH3O2 d. none of the above 16. A 53.00 g sample of strontium reacts with an excess of nitrogen to form strontium nitride. Wha ...
c2 atomic structure f pmh
... A lithium atom can lose one electron to form a lithium ion which can be written (2)+ A fluorine atom can gain one electron to form a fluoride ion. Choose from the list the correct way to write the fluoride ion. ...
... A lithium atom can lose one electron to form a lithium ion which can be written (2)+ A fluorine atom can gain one electron to form a fluoride ion. Choose from the list the correct way to write the fluoride ion. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.