Atomic Structure
... accounting for most of the mass of the atom • The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
... accounting for most of the mass of the atom • The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
Chemistry Powerpoint #5 ATOMIC STRUCTURE THEORIES
... Even today, basic principles of his theory are still true. His model was modified to explain new observations of later chemists ...
... Even today, basic principles of his theory are still true. His model was modified to explain new observations of later chemists ...
Atomic Mass
... His ideas account for the law of conservation of mass - atoms are neither created nor ...
... His ideas account for the law of conservation of mass - atoms are neither created nor ...
Chapter 7: Chemical Formulas and Chemical Compounds
... common names for them do not give any information on the chemical composition. To solve this problem, we give all compounds formulas that give this information. B. Significance of a Chemical Formula 1. For a molecular compound, the chemical formula gives the number of atoms of each element that is p ...
... common names for them do not give any information on the chemical composition. To solve this problem, we give all compounds formulas that give this information. B. Significance of a Chemical Formula 1. For a molecular compound, the chemical formula gives the number of atoms of each element that is p ...
Chapter 8 and 10: Structure of the Atom
... 4. What specific information did Thompson include in his model of the atom that was different from Dalton? 5. Describe Millikan’s oil-drop experiment, the observations he made and the inductive reasoning he used to further the developing model of the atom. 6. Describe Rutherford’s gold foil experime ...
... 4. What specific information did Thompson include in his model of the atom that was different from Dalton? 5. Describe Millikan’s oil-drop experiment, the observations he made and the inductive reasoning he used to further the developing model of the atom. 6. Describe Rutherford’s gold foil experime ...
Ch 6.7 - Explaining the Atom
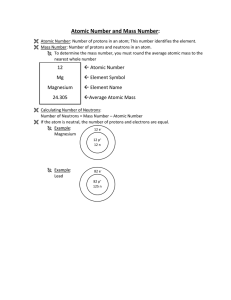
... Evidence of Learning: Students can … - describe and relate atomic number, atomic mass, and mass number. - find the number of neutrons in an atom from its atomic number and atomic ...
... Evidence of Learning: Students can … - describe and relate atomic number, atomic mass, and mass number. - find the number of neutrons in an atom from its atomic number and atomic ...
AP Chemistry
... Law of Multiple Proportions: when two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers ...
... Law of Multiple Proportions: when two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers ...
Atomic Structure
... He asked this question: If you break a piece of matter in half, and then break it in half again, how many breaks will you have to make before you can break it no further? Democritus thought that it ended at some point, a smallest possible bit of matter. He called these basic matter particles, atoms. ...
... He asked this question: If you break a piece of matter in half, and then break it in half again, how many breaks will you have to make before you can break it no further? Democritus thought that it ended at some point, a smallest possible bit of matter. He called these basic matter particles, atoms. ...
Hydrogen—Element #1 - Common Sense Science
... particles is the simplest form of matter that is charge neutral and stable. Spectral Lines of Hydrogen. When “excited” by addition of external energy such as an electric arc, pressure, radiation, etc., hydrogen emits light of specific frequencies and wavelengths. This “line spectra” may reveal prope ...
... particles is the simplest form of matter that is charge neutral and stable. Spectral Lines of Hydrogen. When “excited” by addition of external energy such as an electric arc, pressure, radiation, etc., hydrogen emits light of specific frequencies and wavelengths. This “line spectra” may reveal prope ...
Atoms and Isotopes Worksheet
... 7. The # of protons in the nucleus of an atom is also known as the atomic _________ (mass/ number/ weight/ charge) 8. Which subatomic particles are found in the nucleus? ...
... 7. The # of protons in the nucleus of an atom is also known as the atomic _________ (mass/ number/ weight/ charge) 8. Which subatomic particles are found in the nucleus? ...
The Wizard Test Maker
... Amount of energy that must be absorbed by reactants in 53. their ground states to reach the transition state so that a reaction can occur (A) A (D) D (B) B (E) E (C) C 54. Energy change associated with a mole of gas and ions reacting with water (A) A (D) D (B) B (E) E (C) C 55. The energy change whe ...
... Amount of energy that must be absorbed by reactants in 53. their ground states to reach the transition state so that a reaction can occur (A) A (D) D (B) B (E) E (C) C 54. Energy change associated with a mole of gas and ions reacting with water (A) A (D) D (B) B (E) E (C) C 55. The energy change whe ...
(3.3 × 10!4) + (2.52 × 10!2) = (3.3 × 10!4) × (2.52 × 10!2)
... behavior or a relation that seems always to be the same under the same conditions. Theory: a well-tested, unifying principle that explains a body of facts and the laws based on them. It is capable of suggesting new hypotheses that can be tested experimentally. ...
... behavior or a relation that seems always to be the same under the same conditions. Theory: a well-tested, unifying principle that explains a body of facts and the laws based on them. It is capable of suggesting new hypotheses that can be tested experimentally. ...
Atoms and Light
... 6. Max Planck: Max Planck proposed that light is emitted in discrete packets of energy called “photons.” This idea revolutionized chemistry and physics and launched the field of quantum mechanics. 7. Quantum Model: Bohr’s model of the atom only worked for hydrogen, but other elements also have uni ...
... 6. Max Planck: Max Planck proposed that light is emitted in discrete packets of energy called “photons.” This idea revolutionized chemistry and physics and launched the field of quantum mechanics. 7. Quantum Model: Bohr’s model of the atom only worked for hydrogen, but other elements also have uni ...
History of Atomic Theory
... Named this smallest piece of matter “atomos,” meaning “not to be cut.” ...
... Named this smallest piece of matter “atomos,” meaning “not to be cut.” ...
Science 9
... in a 100-g beaker, a student added 25 g of lead (II) nitrate to 15 g of sodium iodide. In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
... in a 100-g beaker, a student added 25 g of lead (II) nitrate to 15 g of sodium iodide. In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
ELEMENTS AND SYMBOLS
... The number of protons in an atom determines its identity, and is called atomic number (Z). In a neutral atom, the number of protons (+) are equal to the number of electrons (–). Almost all the mass of the atom rests in the nucleus. Therefore the number of protons and neutrons in an atom is cal ...
... The number of protons in an atom determines its identity, and is called atomic number (Z). In a neutral atom, the number of protons (+) are equal to the number of electrons (–). Almost all the mass of the atom rests in the nucleus. Therefore the number of protons and neutrons in an atom is cal ...
Review Worksheet
... a) An electron falls from energy level 3 to energy level 2. What color of visible light is emitted? b) An electron falls from energy level 6 to energy level 3. What is the wavelength of the light emitted? 32. As an electron falls from a higher energy level to a lower one, energy is (absorbed, releas ...
... a) An electron falls from energy level 3 to energy level 2. What color of visible light is emitted? b) An electron falls from energy level 6 to energy level 3. What is the wavelength of the light emitted? 32. As an electron falls from a higher energy level to a lower one, energy is (absorbed, releas ...
S3 Chemistry - eduBuzz.org
... Describe how a covalent bond forms Describe the properties of a covalent compound Explain why noble gases are unreactive State that electrons are found in orbitals of differing shape Predict the bond order by the number of shared pairs of electrons State whether covalent substances form ...
... Describe how a covalent bond forms Describe the properties of a covalent compound Explain why noble gases are unreactive State that electrons are found in orbitals of differing shape Predict the bond order by the number of shared pairs of electrons State whether covalent substances form ...
N5 Chemistry Summary notes 2017
... Atoms of the same element always have the number of protons but the number of electrons can change when a compound is formed. This gives the atom a charge and we call it an ion. Metal atoms form positive ions Non-metal atoms form negative ions. Positive and negative ions are found together in some c ...
... Atoms of the same element always have the number of protons but the number of electrons can change when a compound is formed. This gives the atom a charge and we call it an ion. Metal atoms form positive ions Non-metal atoms form negative ions. Positive and negative ions are found together in some c ...
Learning Outcomes for Chemical Reactions and
... • Describe how a covalent bond forms • Describe the properties of a covalent compound • Explain why noble gases are unreactive • State that electrons are found in orbitals of differing shape • Predict the bond order by the number of shared pairs of electrons • State whether covalent substances form ...
... • Describe how a covalent bond forms • Describe the properties of a covalent compound • Explain why noble gases are unreactive • State that electrons are found in orbitals of differing shape • Predict the bond order by the number of shared pairs of electrons • State whether covalent substances form ...
atoms
... Dalton’s Atomic Theory (experiment based!) 1) All elements are composed of tiny indivisible particles called atoms 2) Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3) Atoms of different elements combine in simple whole-number ratios ...
... Dalton’s Atomic Theory (experiment based!) 1) All elements are composed of tiny indivisible particles called atoms 2) Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3) Atoms of different elements combine in simple whole-number ratios ...
Practice problems for chapter 1, 2 and 3 1) A small amount of salt
... C) +3 D) -5 E) -6 30) Horizontal rows of the periodic table are known as __________. A) periods B) groups C) metalloids D) metals E) nonmetals 31) Elements in Group 7A are known as the __________. A) chalcogens B) alkali metals C) alkaline earth metals D) halogens E) noble gases 32) When a metal and ...
... C) +3 D) -5 E) -6 30) Horizontal rows of the periodic table are known as __________. A) periods B) groups C) metalloids D) metals E) nonmetals 31) Elements in Group 7A are known as the __________. A) chalcogens B) alkali metals C) alkaline earth metals D) halogens E) noble gases 32) When a metal and ...
The Periodic Table
... An atom is the smallest quantity of matter that still retains the properties of matter. An element is a substance that cannot be broken down into two or more simpler substances by any means. Examples: gold, oxygen, helium ...
... An atom is the smallest quantity of matter that still retains the properties of matter. An element is a substance that cannot be broken down into two or more simpler substances by any means. Examples: gold, oxygen, helium ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.