Study Guide
... 10. Who proposed that electrons could behave like waves, as well as like particles? A) Thomson B) Rutherford C) Bohr D) de Broglie E) Heisenberg 11. In the calcium atom represented by the symbol 4020Ca, there are 20 protons, 20 neutrons and 20 electrons. ...
... 10. Who proposed that electrons could behave like waves, as well as like particles? A) Thomson B) Rutherford C) Bohr D) de Broglie E) Heisenberg 11. In the calcium atom represented by the symbol 4020Ca, there are 20 protons, 20 neutrons and 20 electrons. ...
Answers to practice questions
... B) dispersion forces C) hydrogen bonds _____ 30. The shape of the carbon tetrachloride (CCl4) molecule is ___. A) linear B) bent C) tetrahedral D) square *Draw lewis structure: _____ 31. If two elements have similar chemical properties, you would expect them to have A) similar atomic masses B) simil ...
... B) dispersion forces C) hydrogen bonds _____ 30. The shape of the carbon tetrachloride (CCl4) molecule is ___. A) linear B) bent C) tetrahedral D) square *Draw lewis structure: _____ 31. If two elements have similar chemical properties, you would expect them to have A) similar atomic masses B) simil ...
EXAM 3
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in the universe including HUMANS *1. Human body’s most abundant ELEMENTS: carbon [C], oxygen [O], hydrogen [H], and nitrogen [N]; for teeth & BONES = calcium [C ...
... c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in the universe including HUMANS *1. Human body’s most abundant ELEMENTS: carbon [C], oxygen [O], hydrogen [H], and nitrogen [N]; for teeth & BONES = calcium [C ...
Review Booklet
... Outline a brief timeline that describe the experiments with matter from Alchemy to Chemistry Aristotle’s view Matter Made up of ___________________________________________________________________ Earth, Air Fire, Water Investigations by scientists, such as Robert Boyle, in the 1600s confirmed that m ...
... Outline a brief timeline that describe the experiments with matter from Alchemy to Chemistry Aristotle’s view Matter Made up of ___________________________________________________________________ Earth, Air Fire, Water Investigations by scientists, such as Robert Boyle, in the 1600s confirmed that m ...
Goal 1 Study Guide and Practice Problems Fill in the following table
... c. 2-8-3 d. 2-7 e. 2-8-8-2 f. 2-8-5 14. An electron configuration can be effective for describing the number of energy levels and the number of valence electrons for all of the elements. In the quantum mechanical model, what is the maximum number of electrons allowed in each of the first four energy ...
... c. 2-8-3 d. 2-7 e. 2-8-8-2 f. 2-8-5 14. An electron configuration can be effective for describing the number of energy levels and the number of valence electrons for all of the elements. In the quantum mechanical model, what is the maximum number of electrons allowed in each of the first four energy ...
Chapter 4 and 5 study guide 2016-2017
... Filtering or straining can be used to separate mixtures based on __________________________ ...
... Filtering or straining can be used to separate mixtures based on __________________________ ...
Worksheet 8 Notes - Department of Chemistry | Oregon State
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
Introduction to particle physics
... - importance of the relative weights of atoms in obtaining the composition of other substances Law of multiple proportions: “if substance A combines with substance B in two or more ways forming substances C and D, then if mass A is held constant, the masses of B in the various products will be relat ...
... - importance of the relative weights of atoms in obtaining the composition of other substances Law of multiple proportions: “if substance A combines with substance B in two or more ways forming substances C and D, then if mass A is held constant, the masses of B in the various products will be relat ...
Development of the Atomic Theory Electron Cloud Model The
... © Fall 2005, Pflugerville ISD, 8th Grade ...
... © Fall 2005, Pflugerville ISD, 8th Grade ...
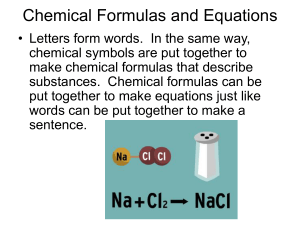
Chemical Formulas and Equations
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
Investigating Atoms and Atomic Theory
... more respected, (and ultimately wrong) theory. Aristotle and Plato favored the earth, fire, air and water approach to the nature of matter. Their ideas held sway because of their eminence as philosophers. The atomos idea was buried for approximately 2000 years. ...
... more respected, (and ultimately wrong) theory. Aristotle and Plato favored the earth, fire, air and water approach to the nature of matter. Their ideas held sway because of their eminence as philosophers. The atomos idea was buried for approximately 2000 years. ...
Presentation
... are presented in the form of a mass spectrum. The horizontal axis shows the mass/charge ratio of the different ions, and the relative abundance of the ions is shown on the vertical axis. Ex. The mass spectrum of gallium shows that in a sample of 100 atoms, 60 have a mass of 69, and 40 have a mas ...
... are presented in the form of a mass spectrum. The horizontal axis shows the mass/charge ratio of the different ions, and the relative abundance of the ions is shown on the vertical axis. Ex. The mass spectrum of gallium shows that in a sample of 100 atoms, 60 have a mass of 69, and 40 have a mas ...
Chapter 2 Atoms and Elements
... for (or at least related to) the chemical periodicity in the elements. When he noted this discrepancy in the periodic table, he said that the physical and chemical properties of these elements dictated their placement in the table and this discrepancy showed that the masses of these two elements mus ...
... for (or at least related to) the chemical periodicity in the elements. When he noted this discrepancy in the periodic table, he said that the physical and chemical properties of these elements dictated their placement in the table and this discrepancy showed that the masses of these two elements mus ...
Structure of the atom
... Each proton has a mass of how many atomic mass units (amu)? (p. 319) Each neutron has a mass of how many atomic mass units (amu)? (p. 319) How does the mass of an electron compare to protons and neutrons? (p. 320) An atom is neutral if the number An atom is neutral if the number of protons and of pr ...
... Each proton has a mass of how many atomic mass units (amu)? (p. 319) Each neutron has a mass of how many atomic mass units (amu)? (p. 319) How does the mass of an electron compare to protons and neutrons? (p. 320) An atom is neutral if the number An atom is neutral if the number of protons and of pr ...
Ch. 07 Notes ch7notes
... Formulas can be used to calculate Molar Masses • From formulas we can tell what elements (or ions) are present and in what quantities. • Molar masses of individual elements (found on the periodic table) are summed to determine molar masses of molecular compounds. Calculating the molar mass of a comp ...
... Formulas can be used to calculate Molar Masses • From formulas we can tell what elements (or ions) are present and in what quantities. • Molar masses of individual elements (found on the periodic table) are summed to determine molar masses of molecular compounds. Calculating the molar mass of a comp ...
Give reasons for the following: (i) Bond enthalpy of F2
... Bond enthalpy of F2 is lower than that of Cl2 because F atom is small in size and due to this the electron-electron repulsions between the lone pairs of F-F are very large. Thus, the bond dissociation energy of F2 is lower than that of Cl2. (ii) PH3 has lower boiling point than NH3 because NH3 molec ...
... Bond enthalpy of F2 is lower than that of Cl2 because F atom is small in size and due to this the electron-electron repulsions between the lone pairs of F-F are very large. Thus, the bond dissociation energy of F2 is lower than that of Cl2. (ii) PH3 has lower boiling point than NH3 because NH3 molec ...
NOTES Atomic Structure Number Mass.docx
... classification system groups similar things together and keeps different things apart. Like in your binder – English is in one section, Math in another, Science in another, and so on. Classification must be clear and practical for the people who are using it. If the basis for sorting is carefully ch ...
... classification system groups similar things together and keeps different things apart. Like in your binder – English is in one section, Math in another, Science in another, and so on. Classification must be clear and practical for the people who are using it. If the basis for sorting is carefully ch ...
Atomic Structure Subatomic Particles Atoms are made up of even
... Atomic number = number of protons in an atom For atoms that are electrically neutral, the number of protons = the number of electrons For ions, the number of protons ≠ the number of electrons Cations have less electrons than an electrically neutral atom Anions have more electrons than an electricall ...
... Atomic number = number of protons in an atom For atoms that are electrically neutral, the number of protons = the number of electrons For ions, the number of protons ≠ the number of electrons Cations have less electrons than an electrically neutral atom Anions have more electrons than an electricall ...
Topic_7_atomic_and_nuclear_energy_IB_problems
... Explain why the temperature and pressure of the gases in the Sun’s core must both be very high for it to produce its radiant energy. High temperature: ............................................................................................ ...
... Explain why the temperature and pressure of the gases in the Sun’s core must both be very high for it to produce its radiant energy. High temperature: ............................................................................................ ...
CHM 103 Lecture 5 S07
... • an element is electrically neutral; the net charge of an atom is zero. • has an equal number of protons and electrons. number of protons = number of electrons Aluminum has 13 protons and 13 electrons. The net charge is zero. ...
... • an element is electrically neutral; the net charge of an atom is zero. • has an equal number of protons and electrons. number of protons = number of electrons Aluminum has 13 protons and 13 electrons. The net charge is zero. ...
CHEM_S1CourseReview_2011
... What rules must be obeyed to safely conduct an experiment? What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How ...
... What rules must be obeyed to safely conduct an experiment? What are the components of a good scientific experiment? What rules must be obeyed to safely conduct an experiment? Why are significant figures important to chemists? What is the best method/graph to represent specific data? How ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.