Atoms, Ions, and the Periodic Table
... Different kinds of atoms have different sizes and shapes. Different properties of matter are due to the differences in size, shape, and movement of atoms. Democritus’ ideas, though correct, were widely rejected by his peers, most notably Aristotle (384-322 BC). Aristotle was a very influential Greek ...
... Different kinds of atoms have different sizes and shapes. Different properties of matter are due to the differences in size, shape, and movement of atoms. Democritus’ ideas, though correct, were widely rejected by his peers, most notably Aristotle (384-322 BC). Aristotle was a very influential Greek ...
How many electrons are present in a chromium
... Tungsten (W) consists of two isotopes. 183W has a mass of 183.26 amu and 184W has a mass of 184.72 amu. Determine the abundance of each isotope of ...
... Tungsten (W) consists of two isotopes. 183W has a mass of 183.26 amu and 184W has a mass of 184.72 amu. Determine the abundance of each isotope of ...
Build An Atom - ChemConnections
... Neutral atoms have ________________________________ protons and electrons. Positive ions have ________________________________ protons than electrons. Negative ions have _______________________________ protons than electrons. ...
... Neutral atoms have ________________________________ protons and electrons. Positive ions have ________________________________ protons than electrons. Negative ions have _______________________________ protons than electrons. ...
Oxidation Numbers and Ionic Compounds
... 5. Subtract the number of electrons already used for the single bonds; two for each bond. 6. Distribute the remaining electrons in pairs around the atoms, trying to satisfy the octet rule. Assign them to the most electronegative atom first. 7. If you run out of electrons before all atoms have an oct ...
... 5. Subtract the number of electrons already used for the single bonds; two for each bond. 6. Distribute the remaining electrons in pairs around the atoms, trying to satisfy the octet rule. Assign them to the most electronegative atom first. 7. If you run out of electrons before all atoms have an oct ...
How many electrons are present in a chromium
... Tungsten (W) consists of two isotopes. 183W has a mass of 183.26 amu and 184W has a mass of 184.72 amu. Determine the abundance of each isotope of ...
... Tungsten (W) consists of two isotopes. 183W has a mass of 183.26 amu and 184W has a mass of 184.72 amu. Determine the abundance of each isotope of ...
4.1 Defining the Atom
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
Chapters 19 & 20
... range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative elements are in s or p orbitals. Metals tend to lose their valence electrons to form cations with a co ...
... range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative elements are in s or p orbitals. Metals tend to lose their valence electrons to form cations with a co ...
Class 9 CBSE Test paper Solved Chapter 3: Structure of...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
... and electronic configuration is 2,8,2. It can lose 2 electrons to get octet configuration thus its valency is 2. Oxygen has atomic number 8 and its electronic configuration is 2, 6. It can gain 2 electrons to get octet configuration thus its valency is 8-6=2 (iii) The atomic number is equal to numbe ...
Chapter 7
... chemical reaction. • It may change form (solid to liquid or gas). • If 1 atom of Carbon goes into a reaction, 1 atom of carbon must come out. It can’t be lost or multiplied. ...
... chemical reaction. • It may change form (solid to liquid or gas). • If 1 atom of Carbon goes into a reaction, 1 atom of carbon must come out. It can’t be lost or multiplied. ...
Stoichiometry 2
... A molecule can be considered the ‘sum of its parts’ (atoms). Thus, simply add the masses of all the atoms in a singe molecule to find the molecule’s mass (formula weight) in amu Determine formula weights for the following molecular materials: Name ...
... A molecule can be considered the ‘sum of its parts’ (atoms). Thus, simply add the masses of all the atoms in a singe molecule to find the molecule’s mass (formula weight) in amu Determine formula weights for the following molecular materials: Name ...
word-doc Practice for the final exam!
... How many unpaired electrons are there in the Lewis structures of a N3- ion? a. 0 b. 1 c. 2 d. 3 e. This cannot be predicted. ...
... How many unpaired electrons are there in the Lewis structures of a N3- ion? a. 0 b. 1 c. 2 d. 3 e. This cannot be predicted. ...
Chapter 2
... ¾ Colorless, diatomic (H2) gas with very low melting point and low density. 9 Reacts with nonmetals to form molecular compounds (Example: HCl and H2O) ¾ Reacts with metals to form hydrides. Metal hydrides react with water to form H2 gas. ¾ HX dissolves in water to form acids. ...
... ¾ Colorless, diatomic (H2) gas with very low melting point and low density. 9 Reacts with nonmetals to form molecular compounds (Example: HCl and H2O) ¾ Reacts with metals to form hydrides. Metal hydrides react with water to form H2 gas. ¾ HX dissolves in water to form acids. ...
Periodic Table Vocab page 7
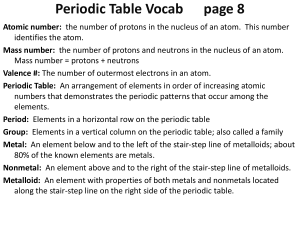
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
MATTER-Ch. 3-homogeneous vs. heterogeneous, elements
... ratios. This evidence supports the law of a. conservation of mass. c. definite composition. b. multiple proportions. d. mass action. ____ 17. According to the law of conservation of mass, when sodium, hydrogen, and oxygen react to form a compound, the mass of the compound is ____ the sum of the mass ...
... ratios. This evidence supports the law of a. conservation of mass. c. definite composition. b. multiple proportions. d. mass action. ____ 17. According to the law of conservation of mass, when sodium, hydrogen, and oxygen react to form a compound, the mass of the compound is ____ the sum of the mass ...
- Chapter 7 - Periodic Properties of the Elements
... Knowing the atomic radii allows the estimation of the bond lengths between different elements in molecules. In the compound CCl4 the measured length of C-Cl bond is 1.77 A° which is very close to the sum of (0.77A°+ 0.99 A°) for C and Cl respectively ...
... Knowing the atomic radii allows the estimation of the bond lengths between different elements in molecules. In the compound CCl4 the measured length of C-Cl bond is 1.77 A° which is very close to the sum of (0.77A°+ 0.99 A°) for C and Cl respectively ...
Chemistry Final Exam Review 2006-2007
... 3. In metallic bonding, the valence electrons of all 12. In nonpolar covalent bonds, valence electrons are atoms are shared in: a. Equally shared a. A nonpolar covalent bond b. Unequally shared b. An electron sea c. Destroyed c. A polar covalent bond d. transferred d. Transferred to metallic ions 13 ...
... 3. In metallic bonding, the valence electrons of all 12. In nonpolar covalent bonds, valence electrons are atoms are shared in: a. Equally shared a. A nonpolar covalent bond b. Unequally shared b. An electron sea c. Destroyed c. A polar covalent bond d. transferred d. Transferred to metallic ions 13 ...
The History of the Atom Web quest
... Useful Sites Dalton site at Frostburg University http://antoine.frostburg.edu/chem/senese/101/atoms/dalton.shtml Guch's explanation of the difference between the two laws http://misterguch.brinkster.net/q14.html Dalton's Atomic Theory http://dl.clackamas.cc.or.us/ch104-04/dalton's.htm More on Dalton ...
... Useful Sites Dalton site at Frostburg University http://antoine.frostburg.edu/chem/senese/101/atoms/dalton.shtml Guch's explanation of the difference between the two laws http://misterguch.brinkster.net/q14.html Dalton's Atomic Theory http://dl.clackamas.cc.or.us/ch104-04/dalton's.htm More on Dalton ...
CHM 103 Lecture 5 S07
... Photosynthesis - carbon in glucose comes from CO2 Calcium - uptake is 90% efficient in children; 40% efficient in adults Zinc - uptake by trees in winter 2 ft/day ...
... Photosynthesis - carbon in glucose comes from CO2 Calcium - uptake is 90% efficient in children; 40% efficient in adults Zinc - uptake by trees in winter 2 ft/day ...
The purpose of this packet is to prepare you for the Biology Course
... You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particl ...
... You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particl ...
4.1 Early Theories of Matter The Philosophers Democritus – Greek
... Was more popular John Dalton –English Schoolteacher (1766-1844) Revived Democritus’ ideas along with research from other prominent scientists of the time Borrowed concept of atomos from Democritus Borrowed Law of Conservation of Matter from Antoine Lavoisier Borrowed Law of Definite Propor ...
... Was more popular John Dalton –English Schoolteacher (1766-1844) Revived Democritus’ ideas along with research from other prominent scientists of the time Borrowed concept of atomos from Democritus Borrowed Law of Conservation of Matter from Antoine Lavoisier Borrowed Law of Definite Propor ...
MISE - Physical Basis of Chemistry
... the ratio of atomic weights can become individual values. Since hydrogen was believed to be the lightest element , H was assigned the weight of “1” and all other atomic weights were determined relative to the ratio with hydrogen. A lot of history intervened - such as isotopes, i.e., atoms of the sam ...
... the ratio of atomic weights can become individual values. Since hydrogen was believed to be the lightest element , H was assigned the weight of “1” and all other atomic weights were determined relative to the ratio with hydrogen. A lot of history intervened - such as isotopes, i.e., atoms of the sam ...
Bohr Atomic Model - Flinn Scientific
... Through the years, significant progress has been made in our knowledge of the atom. Atoms were originally described as the smallest particles of matter. The discoveries, in turn, of the electron, proton and neutron destroyed the notion of the indivisible atom. Knowledge of the subatomic particle mak ...
... Through the years, significant progress has been made in our knowledge of the atom. Atoms were originally described as the smallest particles of matter. The discoveries, in turn, of the electron, proton and neutron destroyed the notion of the indivisible atom. Knowledge of the subatomic particle mak ...
Atomic Structure
... Heart cell rhythm depends on the opening and closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions ike potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or a ...
... Heart cell rhythm depends on the opening and closing of a complex series of valves on the cell membrane, called ion channels. Some valves let certain ions ike potassium (K+) flow out, others let different ions like sodium (Na+) flow in. There are also pumps that actively move ions one direction or a ...
The Atom
... The Atom The model of the atom has evolved from Dalton's concept of it as a "solid billiard ball" to a highly complicated model. At first it was felt that it could not be broken down. It was soon discovered the atom could be broken down into 3 sub-atomic particles known as electrons, protons and neu ...
... The Atom The model of the atom has evolved from Dalton's concept of it as a "solid billiard ball" to a highly complicated model. At first it was felt that it could not be broken down. It was soon discovered the atom could be broken down into 3 sub-atomic particles known as electrons, protons and neu ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.