Review of Analytical Methods Part 1: Spectrophotometry
... – Glutathione (Glutathione reductase also uses NADPH as a cofactor) – Adenosine kinase phosphorylates ADP to ATP (fluoride ion inhibits AK activity – Calcium ion may inhibit CK activity, since the enzyme is Mg++-dependent. ...
... – Glutathione (Glutathione reductase also uses NADPH as a cofactor) – Adenosine kinase phosphorylates ADP to ATP (fluoride ion inhibits AK activity – Calcium ion may inhibit CK activity, since the enzyme is Mg++-dependent. ...
Sample Questions Chapters 9-10
... ____ 38. Cellular respiration harvests the most chemical energy from which of the following? a. substrate-level phosphorylation b. chemiosmotic phosphorylation c. converting oxygen to ATP d. transferring electrons from organic molecules to pyruvate e. generating carbon dioxide and oxygen in the elec ...
... ____ 38. Cellular respiration harvests the most chemical energy from which of the following? a. substrate-level phosphorylation b. chemiosmotic phosphorylation c. converting oxygen to ATP d. transferring electrons from organic molecules to pyruvate e. generating carbon dioxide and oxygen in the elec ...
Position versus Substrate
... the glycosylated product compared to the glycoside. From the few known glycosylated plantderived and biologically active compounds like the saponins, the attachment of several hydrophilic sugars, rather than of one specific sugar is responsible for the observed detrimental effect on membranes (Osbou ...
... the glycosylated product compared to the glycoside. From the few known glycosylated plantderived and biologically active compounds like the saponins, the attachment of several hydrophilic sugars, rather than of one specific sugar is responsible for the observed detrimental effect on membranes (Osbou ...
Cellular Respiration
... Hydrogen carriers such as shuttle electrons in redox reactions Enzymes remove electrons from glucose molecules and transfer them to a coenzyme OXIDATION Dehydrogenase and NAD+ ...
... Hydrogen carriers such as shuttle electrons in redox reactions Enzymes remove electrons from glucose molecules and transfer them to a coenzyme OXIDATION Dehydrogenase and NAD+ ...
video slide
... • The electron transport chain – Passes electrons in a series of steps instead of in one explosive reaction – Uses the energy from the electron transfer to form ATP ...
... • The electron transport chain – Passes electrons in a series of steps instead of in one explosive reaction – Uses the energy from the electron transfer to form ATP ...
Carbohydrate Metabolism-1
... 1. Glycolysis means oxidation of glucose to give pyruvate (in the presence of oxygen) or lactate (in the absence of oxygen). ...
... 1. Glycolysis means oxidation of glucose to give pyruvate (in the presence of oxygen) or lactate (in the absence of oxygen). ...
Anaerobic Respiration
... A major advantage of aerobic respiration is the amount of energy it releases. Without oxygen, organisms can just split glucose into two molecules of pyruvate. This releases only enough energy to make two ATP molecules. With oxygen, organisms can break down glucose all the way to carbon dioxide. This ...
... A major advantage of aerobic respiration is the amount of energy it releases. Without oxygen, organisms can just split glucose into two molecules of pyruvate. This releases only enough energy to make two ATP molecules. With oxygen, organisms can break down glucose all the way to carbon dioxide. This ...
video slide - Somers Public Schools
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
Glycogen Metabolism and Gluconeogenesis
... Ga is irreversibly modified by addition of ADP-ribosyl group; Modified Gα can bind GTP but cannot hydrolyze it ). As a result, there is an excessive, nonregulated rise in the intracellular cAMP level (100 fold or more), which causes a large efflux of Na+ and water into the gut. Pertussis (whooping c ...
... Ga is irreversibly modified by addition of ADP-ribosyl group; Modified Gα can bind GTP but cannot hydrolyze it ). As a result, there is an excessive, nonregulated rise in the intracellular cAMP level (100 fold or more), which causes a large efflux of Na+ and water into the gut. Pertussis (whooping c ...
Chapter 9
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through channels in ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP ...
APB Chapter 9 Cellular Respiration: Harvesting Chemical Energy
... These steps can be divided into two phases. 1. ______________________________________________________________________, the cell spends ATP. 2. ______________________________________, this investment is repaid with interest. ATP is produced by substrate-level phosphorylation, and NAD+ is reduced to N ...
... These steps can be divided into two phases. 1. ______________________________________________________________________, the cell spends ATP. 2. ______________________________________, this investment is repaid with interest. ATP is produced by substrate-level phosphorylation, and NAD+ is reduced to N ...
Biol 1406 notes Ch 9 8thed
... Electrons are passed to increasingly electronegative molecules in the chain until they reduce oxygen, the most electronegative receptor. Each “downhill” carrier is more electronegative than, and thus capable of oxidizing, its “uphill” neighbor, with oxygen at the bottom of the chain. The elec ...
... Electrons are passed to increasingly electronegative molecules in the chain until they reduce oxygen, the most electronegative receptor. Each “downhill” carrier is more electronegative than, and thus capable of oxidizing, its “uphill” neighbor, with oxygen at the bottom of the chain. The elec ...
Glucose-6-P to Fructose-6-P
... – If O2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation – In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis ...
... – If O2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation – In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis ...
Glucose-6-P to Fructose-6-P
... – If O2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation – In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis ...
... – If O2 is available, NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation – In anaerobic conditions, NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis ...
of Glycolysis
... • Phosphofructokinase‐ major control point; first enzyme “unique” to glycolysis • Pyruvate kinase •Phosphofructokinase responds to changes in: • Energy state of the cell (high ATP levels inhibit) • H+ concentration (high lactate levels inhibit) • Availability of alternate fuels such as fatty acids, ...
... • Phosphofructokinase‐ major control point; first enzyme “unique” to glycolysis • Pyruvate kinase •Phosphofructokinase responds to changes in: • Energy state of the cell (high ATP levels inhibit) • H+ concentration (high lactate levels inhibit) • Availability of alternate fuels such as fatty acids, ...
Application Project Unit 1
... industries (e.g., the production of dairy products; breadmaking; the use of enzymes to control reaction rates in pharmaceuticals) [AI, C]; ...
... industries (e.g., the production of dairy products; breadmaking; the use of enzymes to control reaction rates in pharmaceuticals) [AI, C]; ...
lecture 6 ppt
... Given an equation, particularly that for cellular respiration, determine which molecules are oxidized and reduced ...
... Given an equation, particularly that for cellular respiration, determine which molecules are oxidized and reduced ...
Regulation of metabolic pathways at the cellular level
... Overview of the lecture • Overview of regulation of metabolic pathways – regulation of respiratory chain and aerobic phosphorylation – regulation of the Krebs cycle – regulation of the oxidative decarboxylation of pyruvate – regulation of glycolysis and gluconeogenesis – regulation of glycogen meta ...
... Overview of the lecture • Overview of regulation of metabolic pathways – regulation of respiratory chain and aerobic phosphorylation – regulation of the Krebs cycle – regulation of the oxidative decarboxylation of pyruvate – regulation of glycolysis and gluconeogenesis – regulation of glycogen meta ...
Chapter 6 – How Cells Harvest Chemical Energy Standard 1.g
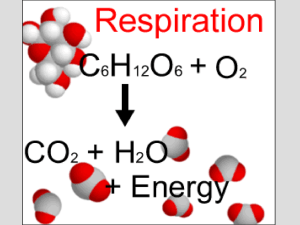
... The process uses O2 and releases CO2 and H2O ...
... The process uses O2 and releases CO2 and H2O ...
H +
... enters glycolysis; fatty acids are converted to acetyl CoA and enter the citric acid cycle ...
... enters glycolysis; fatty acids are converted to acetyl CoA and enter the citric acid cycle ...
Pyruvate Glucose - School of Medicine
... All the DHAP is converted to glyceraldehyde 3phosphate. Although, the reaction is reversible it is shifted to the right since glyceraldehyde 3phosphate is a substrate for the next reactions of glycolysis. Thus, both 3-carbon fragments are subsequently oxidized. ...
... All the DHAP is converted to glyceraldehyde 3phosphate. Although, the reaction is reversible it is shifted to the right since glyceraldehyde 3phosphate is a substrate for the next reactions of glycolysis. Thus, both 3-carbon fragments are subsequently oxidized. ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.