glucose - WordPress.com
... AMP and ADP are activators. As ATP is consumed, ADP and sometimes AMP levels build up, triggering the need for more ATP. The enzyme is highly regulated by ATP. If there is a lot of ATP in the cell, then glycolysis is not necessary.. ATP will build at an allosteric site and inhibit binding of F6-P. ...
... AMP and ADP are activators. As ATP is consumed, ADP and sometimes AMP levels build up, triggering the need for more ATP. The enzyme is highly regulated by ATP. If there is a lot of ATP in the cell, then glycolysis is not necessary.. ATP will build at an allosteric site and inhibit binding of F6-P. ...
Carbohydrate Metabolism
... c) Pyruvate: which can be used in synthesis of amino acid alanine. 4. Aerobic glycolysis provides the mitochondria with pyruvate, which gives acetyl CoA Krebs' cycle. ...
... c) Pyruvate: which can be used in synthesis of amino acid alanine. 4. Aerobic glycolysis provides the mitochondria with pyruvate, which gives acetyl CoA Krebs' cycle. ...
Biosynthesis of Isoprenoids
... An interesting feature of the monoterpene synthases is the ability of these enzymes to produce more than one product; for example, pinene synthase from several plant sources produces both α- and β-pinene. The pinenes are among the most common monoterpenes produced by plants and are principal compone ...
... An interesting feature of the monoterpene synthases is the ability of these enzymes to produce more than one product; for example, pinene synthase from several plant sources produces both α- and β-pinene. The pinenes are among the most common monoterpenes produced by plants and are principal compone ...
Chapter 3
... • transported into _______________oxidized via Krebs cycle • Released into the blood stream where it can be used by other muscles (e.g., heart, other muscles) • Released into the blood stream where it can be used by the liver (production of blood glucose). ...
... • transported into _______________oxidized via Krebs cycle • Released into the blood stream where it can be used by other muscles (e.g., heart, other muscles) • Released into the blood stream where it can be used by the liver (production of blood glucose). ...
CH - IS MU
... Threonine - by splitting gives glycine that may give serine. Cysteine also gives pyruvate by deamination and desulfuration (see "Amino acids containing sulfur"), as well as tryptophan that after transformation to kynurenin releases alanine ...
... Threonine - by splitting gives glycine that may give serine. Cysteine also gives pyruvate by deamination and desulfuration (see "Amino acids containing sulfur"), as well as tryptophan that after transformation to kynurenin releases alanine ...
CELLULAR RESPIRATION
... complex I to IV in the electron transport chain, they are coupled to ATP synthase (complex V) for the production of ATP from ADP and inorganic phosphate. The number of ATP molecules synthesized depends on the nature of the electron donor. Oxidation of one molecule of NADH gives rise to 3 molecules o ...
... complex I to IV in the electron transport chain, they are coupled to ATP synthase (complex V) for the production of ATP from ADP and inorganic phosphate. The number of ATP molecules synthesized depends on the nature of the electron donor. Oxidation of one molecule of NADH gives rise to 3 molecules o ...
metalloenzyme_1
... Certain metals have long been recognized to have important biological functions primarily as a consequence of nutritional investigations. Thus, the absence of a specific, essential metal from the diet of an organism invariably leads to a deficiency state characterized by metabolic abnormalities with ...
... Certain metals have long been recognized to have important biological functions primarily as a consequence of nutritional investigations. Thus, the absence of a specific, essential metal from the diet of an organism invariably leads to a deficiency state characterized by metabolic abnormalities with ...
Clinical Biochemistry
... (where m could be different from n). Carbohydrates are technically hydrates of carbon structurally it is more accurate to view them as polyhydroxy aldehydes and ketones. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In g ...
... (where m could be different from n). Carbohydrates are technically hydrates of carbon structurally it is more accurate to view them as polyhydroxy aldehydes and ketones. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In g ...
FOR ENZYMES THE LIMITS FOR LIFE DEFINE THE LIMITS
... nutrients and temperature, they can undergo cell division to produce two cells in about 20 min. Since nutrients are at an optimum, this means that the concentration of the substrate is not a limiting variable. However, any necessary chemical reaction must normally occur many times within a cell’s li ...
... nutrients and temperature, they can undergo cell division to produce two cells in about 20 min. Since nutrients are at an optimum, this means that the concentration of the substrate is not a limiting variable. However, any necessary chemical reaction must normally occur many times within a cell’s li ...
Answer Set 3
... would be simply, ATP + H2O --> ADP + Pi. The energy of ATP hydrolysis would be released as heat. b. Do these results support the notion that bumblebees use futile cycles to generate heat? Not really. For the cycle to generate heat, both enzymes must be functional at the same time in the same cell. c ...
... would be simply, ATP + H2O --> ADP + Pi. The energy of ATP hydrolysis would be released as heat. b. Do these results support the notion that bumblebees use futile cycles to generate heat? Not really. For the cycle to generate heat, both enzymes must be functional at the same time in the same cell. c ...
Microbial Metabolism Lecture 4
... are broken, it can release energy. So we’re recycling this product all the time; we start with ATP, break a bond, release energy, use that energy, and then through the process of metabolism we have to attach the high-phosphate bond back to the ADP to produce, again, a new ATP molecule. Cells can do ...
... are broken, it can release energy. So we’re recycling this product all the time; we start with ATP, break a bond, release energy, use that energy, and then through the process of metabolism we have to attach the high-phosphate bond back to the ADP to produce, again, a new ATP molecule. Cells can do ...
File - John Robert Warner
... • Vitamins are also a necessity for humans; we cannot synthesize them, yet they are critical building blocks for coenzymes. © 2013 Pearson Education, Inc. ...
... • Vitamins are also a necessity for humans; we cannot synthesize them, yet they are critical building blocks for coenzymes. © 2013 Pearson Education, Inc. ...
Isoenzyme Loci in Brown Trout (Salmo Trutta L.)
... Previously reported results on natural populations of brown trout ( S a h o trutta L.) urged further studies using additional electrophoretically detectable loci. Samples of brain, eye, heart, kidney, liver, and muscle from approximately SO specimens from each of two populations and a large number o ...
... Previously reported results on natural populations of brown trout ( S a h o trutta L.) urged further studies using additional electrophoretically detectable loci. Samples of brain, eye, heart, kidney, liver, and muscle from approximately SO specimens from each of two populations and a large number o ...
Document
... • Vitamins are also a necessity for humans; we cannot synthesize them, yet they are critical building blocks for coenzymes. © 2013 Pearson Education, Inc. ...
... • Vitamins are also a necessity for humans; we cannot synthesize them, yet they are critical building blocks for coenzymes. © 2013 Pearson Education, Inc. ...
Glycolysis
... - thus two ATP used in Phase 1 - substrate level phosphorylationATP production by the direct transfer of phosphate from intermediate ___________________ _____________________________23 ...
... - thus two ATP used in Phase 1 - substrate level phosphorylationATP production by the direct transfer of phosphate from intermediate ___________________ _____________________________23 ...
Cobalt Biology Discussion - 1-29-15
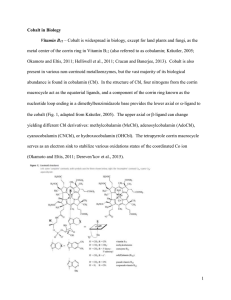
... hydratase catalyzes hydration of nitriles to amides, and is a key enzyme involved in the metabolism of toxic compounds (Kobayashi et al., 1992). [4] Glucose isomerase catalyzes the reversible isomeration of D-glucose to D-fructose and is one of the most highly used enzymes in industry (Bhosale et al ...
... hydratase catalyzes hydration of nitriles to amides, and is a key enzyme involved in the metabolism of toxic compounds (Kobayashi et al., 1992). [4] Glucose isomerase catalyzes the reversible isomeration of D-glucose to D-fructose and is one of the most highly used enzymes in industry (Bhosale et al ...
enzymes
... The increase in highest quality first press juice yield can go up to at least 10% and the pressing time can be reduced by 20‐50% as a result of the presence of enzymes. ...
... The increase in highest quality first press juice yield can go up to at least 10% and the pressing time can be reduced by 20‐50% as a result of the presence of enzymes. ...
Metabolic Acidosis
... • Net production of L-lactic acid occurs when the body must regenerate ATP without oxygen • 1 H+ is produced per ATP regenerated from glucose • Because a patient will need to regenerate 72 mmol of ATP per minutes, As much as 72 mmol/min of H+ can be produced in case of anoxia • 2ATP2 ADP + 2 Pi + b ...
... • Net production of L-lactic acid occurs when the body must regenerate ATP without oxygen • 1 H+ is produced per ATP regenerated from glucose • Because a patient will need to regenerate 72 mmol of ATP per minutes, As much as 72 mmol/min of H+ can be produced in case of anoxia • 2ATP2 ADP + 2 Pi + b ...
- Wiley Online Library
... NADH, but the enzymatic reaction is very complex due to interference of numerous side reactions [18]. Allyl and cinnamyl alcohol are, however, excellent substrates; kinetic constants for the latter alcohol are: V1 = 133 s31 and V1 /KB = 29 mM31 s31 , at pH 8.2, 25‡C [19]. (S)-(+)-Butan-2-ol is a muc ...
... NADH, but the enzymatic reaction is very complex due to interference of numerous side reactions [18]. Allyl and cinnamyl alcohol are, however, excellent substrates; kinetic constants for the latter alcohol are: V1 = 133 s31 and V1 /KB = 29 mM31 s31 , at pH 8.2, 25‡C [19]. (S)-(+)-Butan-2-ol is a muc ...
Document
... • DFP is an organic phosphate that inactivates serine proteases, it can react with the active site serine (Ser-195) of enzyme to form DFP-E. • These inhibitors are toxic because they inhibit acetylcholin esterase (a serine protease that hydrolyzes the neurotransmitter acetylcholine). • Such organoph ...
... • DFP is an organic phosphate that inactivates serine proteases, it can react with the active site serine (Ser-195) of enzyme to form DFP-E. • These inhibitors are toxic because they inhibit acetylcholin esterase (a serine protease that hydrolyzes the neurotransmitter acetylcholine). • Such organoph ...
lecture notes-enzyme-web
... - the binding force between enzyme and carrier is so strong that no leakage of the enzymes occurs. ...
... - the binding force between enzyme and carrier is so strong that no leakage of the enzymes occurs. ...
The Biochemistry of Malic Acid Metabolism by Wine Yeasts
... and in the presence of high concentrations of glucose, cells of S. cerevisiae do not have functional mitochondria (Fraenkel, 1982), but cytosolic enzymes similar to those in the TCA cycle produce the necessary biosynthetic intermediates. The enzymatic reactions of the TCA cycle include the hydration ...
... and in the presence of high concentrations of glucose, cells of S. cerevisiae do not have functional mitochondria (Fraenkel, 1982), but cytosolic enzymes similar to those in the TCA cycle produce the necessary biosynthetic intermediates. The enzymatic reactions of the TCA cycle include the hydration ...
Specific Activities of Enzymes of the Serine Pathway of Carbon
... and the optima were respectively pH6.0, 7.0 and 7.5. Table 1 shows that both organisms when grown on methylamine possessed elevated activities of the enzymes serine hydroxymethyltransferase, hydroxypyruvate reductase, malate dehydrogenase, phosphoenolpyruvate carboxylase, serineglyoxylate aminotrans ...
... and the optima were respectively pH6.0, 7.0 and 7.5. Table 1 shows that both organisms when grown on methylamine possessed elevated activities of the enzymes serine hydroxymethyltransferase, hydroxypyruvate reductase, malate dehydrogenase, phosphoenolpyruvate carboxylase, serineglyoxylate aminotrans ...
PowerPoint
... D-Lactate: Internal Sources In the gut, glucose is metabolized by flora to lactate: l-Lactate d-Lactate ...
... D-Lactate: Internal Sources In the gut, glucose is metabolized by flora to lactate: l-Lactate d-Lactate ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.