2/25/11 QUANTUM MECHANICS II (524) PROBLEM SET 6 (hand in
... 22) (20 points) The hydrogen atom nucleus is a proton with spin I = 1/2. a) In the notation of the preceding problem, what are the possible values of the quantum numbers J and F for a hydrogen atom in the 2p level? b) Use the notation {|n`mi} for the eigenstates of the “simple” hydrogen Hamiltonian ...
... 22) (20 points) The hydrogen atom nucleus is a proton with spin I = 1/2. a) In the notation of the preceding problem, what are the possible values of the quantum numbers J and F for a hydrogen atom in the 2p level? b) Use the notation {|n`mi} for the eigenstates of the “simple” hydrogen Hamiltonian ...
Modern Physics - Tarleton State University
... Man, were they in for a surprise. Several of them actually. Modern physics is the story of these surprises (quantum mechanics and special and general relativity), surprises—revolutions, actually—that have changed the world beyond all recognition. The purpose of this course is to introduce you to all ...
... Man, were they in for a surprise. Several of them actually. Modern physics is the story of these surprises (quantum mechanics and special and general relativity), surprises—revolutions, actually—that have changed the world beyond all recognition. The purpose of this course is to introduce you to all ...
CH7 handout is here.
... o Heisenberg's Uncertainty Principle 7.4The Quantum-Mechanical Model of the Atom o Atomic Orbital and Probable Location of the Electron o Quantum Numbers of an Orbital o Quantum Numbers and Energy Levels o Shapes of Atomic Orbitals o The Special Case of the H Atom Concepts and Skills to Review Befor ...
... o Heisenberg's Uncertainty Principle 7.4The Quantum-Mechanical Model of the Atom o Atomic Orbital and Probable Location of the Electron o Quantum Numbers of an Orbital o Quantum Numbers and Energy Levels o Shapes of Atomic Orbitals o The Special Case of the H Atom Concepts and Skills to Review Befor ...
Chapter7Part3
... - the electron is moving so fast and it has such a its position and its velocity at small mass, that its path cannot be predicted various times we think of the ball as moving along a continuous path Heisenberg’s Uncertainty Principle states that for particles of very small mass and moving at high sp ...
... - the electron is moving so fast and it has such a its position and its velocity at small mass, that its path cannot be predicted various times we think of the ball as moving along a continuous path Heisenberg’s Uncertainty Principle states that for particles of very small mass and moving at high sp ...
Rutherford`s Atomic Model
... Which of the following modifications to Rutherford’s atomic model can help explain why atoms in gases emit electromagnetic radiation of some characteristic frequencies? A. An atom can only contain certain numbers of nucleons. B. An atom can only contain certain numbers of electrons. C. Electrons orb ...
... Which of the following modifications to Rutherford’s atomic model can help explain why atoms in gases emit electromagnetic radiation of some characteristic frequencies? A. An atom can only contain certain numbers of nucleons. B. An atom can only contain certain numbers of electrons. C. Electrons orb ...
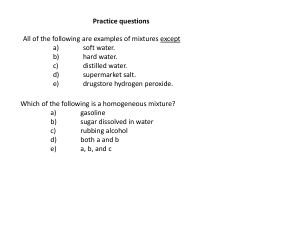
Practice questions
... There are three isotopes of carbon differing with respect to a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... There are three isotopes of carbon differing with respect to a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
PowerPoint Presentation - Duality of Matter
... Just a stream of particles that carries energy with it. A unique entity that exhibits both wave and particle properties. ...
... Just a stream of particles that carries energy with it. A unique entity that exhibits both wave and particle properties. ...
Chapter 2
... 9. Given the following sets of quantum numbers, set up the orbital notation (labeled with the sublevel designation) for the indicated sublevel of electrons and circle the indicated orbital. Assume that electrons fill from more negative to more positive ml values. ...
... 9. Given the following sets of quantum numbers, set up the orbital notation (labeled with the sublevel designation) for the indicated sublevel of electrons and circle the indicated orbital. Assume that electrons fill from more negative to more positive ml values. ...
Define:
... Name the following ions: N3-, P3-, S2104. Write formulas for the following ions: hydroxide, sulfate, sulfite, nitrate, nitrite, cyanide, ammonium, phosphate, carbonate, and acetate. ...
... Name the following ions: N3-, P3-, S2104. Write formulas for the following ions: hydroxide, sulfate, sulfite, nitrate, nitrite, cyanide, ammonium, phosphate, carbonate, and acetate. ...
final study guide answers - Ponce
... d. Scientific theories summarize patterns found in nature. __B_____4. Why are scientific models important? a. They prove scientific theories. b. They help visualize things that are very complex, very large, or very small. c. They make it harder to understand things. d. They never change. ...
... d. Scientific theories summarize patterns found in nature. __B_____4. Why are scientific models important? a. They prove scientific theories. b. They help visualize things that are very complex, very large, or very small. c. They make it harder to understand things. d. They never change. ...
03-02BohrAtom
... What is the energy of the n = 5 orbital? What is the energy of the n = 3 orbital? What is the wavelength of the photon released from a 5 to 3 transition? (Hydrogen atom) ...
... What is the energy of the n = 5 orbital? What is the energy of the n = 3 orbital? What is the wavelength of the photon released from a 5 to 3 transition? (Hydrogen atom) ...
Ch 2 notes
... 1. qualitative…what does it contain, and 2. quantitative…how much of everything does it contain B) Stoichiometry – composition stoichiometry (this chapter) and reaction stoichiometry (ch 3) 2-1 Atoms and Molecules A) Aristotle v Democritus 1. Early scientists – philosophers/thinkers – NOT experiment ...
... 1. qualitative…what does it contain, and 2. quantitative…how much of everything does it contain B) Stoichiometry – composition stoichiometry (this chapter) and reaction stoichiometry (ch 3) 2-1 Atoms and Molecules A) Aristotle v Democritus 1. Early scientists – philosophers/thinkers – NOT experiment ...
Periodic Properties of the Elements
... Recall that the number of electrons is equal to the atomic number of an element Properties to be considered Atomic Radius (and Ionic Radius) ...
... Recall that the number of electrons is equal to the atomic number of an element Properties to be considered Atomic Radius (and Ionic Radius) ...
atomic number
... • In a neutral atom, # electrons = #protons. • The symbol for an element is simply its 1, 2, or 3 letter abbreviation from the periodic table. One capital letter or one capital letter + one or two lower case letters. • Two atoms that have the same number of protons, but a different number of neutron ...
... • In a neutral atom, # electrons = #protons. • The symbol for an element is simply its 1, 2, or 3 letter abbreviation from the periodic table. One capital letter or one capital letter + one or two lower case letters. • Two atoms that have the same number of protons, but a different number of neutron ...
atoms-chemical
... Atomic structure determines the behavior of an element • Each element consists of unique atoms. • An atom is the smallest unit of matter that still retains the properties of an element. • Atoms are composed of neutrons, protons, and electrons form a cloud around the nucleus. ...
... Atomic structure determines the behavior of an element • Each element consists of unique atoms. • An atom is the smallest unit of matter that still retains the properties of an element. • Atoms are composed of neutrons, protons, and electrons form a cloud around the nucleus. ...
Covalent Bonds - WordPress.com
... The Energy Levels of Electrons • Electrons of atoms participate in reactions • Energy is the capacity to cause change • Potential energy is the energy that matter has because of its location or structure • The electrons of an atom differ in their amounts of potential energy • An electron’s state of ...
... The Energy Levels of Electrons • Electrons of atoms participate in reactions • Energy is the capacity to cause change • Potential energy is the energy that matter has because of its location or structure • The electrons of an atom differ in their amounts of potential energy • An electron’s state of ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.