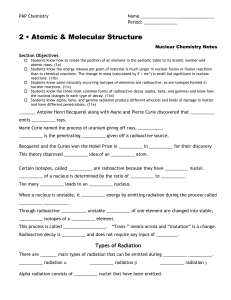
Types of Radiation
... 2 • Atomic & Molecular Structure Nuclear Chemistry Notes Section Objectives Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission re ...
... 2 • Atomic & Molecular Structure Nuclear Chemistry Notes Section Objectives Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission re ...
Average Atomic Mass
... B/G. Be C/H. H 32. Beryllium 33. Boron 34. Helium 35. Hydrogen Write the formula for the compound containing 36. a two to three ratio of iron to oxygen A/F. Fe2O3 B/G. I2O3 C/H. Fe3O2 ...
... B/G. Be C/H. H 32. Beryllium 33. Boron 34. Helium 35. Hydrogen Write the formula for the compound containing 36. a two to three ratio of iron to oxygen A/F. Fe2O3 B/G. I2O3 C/H. Fe3O2 ...
PPT | 187.5 KB - Joint Quantum Institute
... precision level of a part in 1015, accurate knowledge of this shift is more pertinent now that clocks are closing in on the part-per-1018 level of precision New calculations by PFC-supported work at the Joint Quantum Institute and the University of Delaware is the best yet since it includes the most ...
... precision level of a part in 1015, accurate knowledge of this shift is more pertinent now that clocks are closing in on the part-per-1018 level of precision New calculations by PFC-supported work at the Joint Quantum Institute and the University of Delaware is the best yet since it includes the most ...
Atomic masses
... Alpha particles: carry two fundamental units of positive charge and have the same mass as helium atoms. They have +2 charges. Beta particles: are negatively charged particles produced by changes occuring within the nuclei of radioactive atoms and have the same properties as electrons. Gama rays are ...
... Alpha particles: carry two fundamental units of positive charge and have the same mass as helium atoms. They have +2 charges. Beta particles: are negatively charged particles produced by changes occuring within the nuclei of radioactive atoms and have the same properties as electrons. Gama rays are ...
HW 8
... This print-out should have 5 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. Mlib 02 0095 001 10.0 points Which of the following provided evidence that the electrons in atoms are arranged in distinct energy levels? 1. the results of t ...
... This print-out should have 5 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. Mlib 02 0095 001 10.0 points Which of the following provided evidence that the electrons in atoms are arranged in distinct energy levels? 1. the results of t ...
Study On the Capacitance Between Orbitals and Atoms Modeling
... Since 100 year, Niels Bohr suggested his model of the atom. He succeeded only in interpretation of line spectrum of single electron atoms (Hydrogen and ionized Helium) so more general model was still needed. In 1926, Erwin Schrödinger presented his famous equation which described complicated physica ...
... Since 100 year, Niels Bohr suggested his model of the atom. He succeeded only in interpretation of line spectrum of single electron atoms (Hydrogen and ionized Helium) so more general model was still needed. In 1926, Erwin Schrödinger presented his famous equation which described complicated physica ...
A = 27
... #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 (13 e-), one electron promoted from shell 2 to shell ...
... #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 (13 e-), one electron promoted from shell 2 to shell ...
Slide 1 - Southwest High School
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
Chapter4 Nuclear atom - UCF College of Sciences
... An expert is a person who has made all the mistakes that can be made in a very narrow field. Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. ...
... An expert is a person who has made all the mistakes that can be made in a very narrow field. Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. ...
Physics 228, Lecture 11 Monday, February 28, 2005 Bohr Model
... The Rutherford atom would have the electron moving in orbits around the nucleus just as planets revolve around the sun. The classical mechanics of these situations are the same, but Bohr added some quantum mechanical rules. Bohr asserted that of all the infinitely many orbits that classical mechanic ...
... The Rutherford atom would have the electron moving in orbits around the nucleus just as planets revolve around the sun. The classical mechanics of these situations are the same, but Bohr added some quantum mechanical rules. Bohr asserted that of all the infinitely many orbits that classical mechanic ...
First Semester Final - Review Questions
... 41. Why is it necessary to perform multiple trials of a scientific experiment? 42. What type of instrument is best for measuring mass, volume, and length? 43. How is the uncertainty of an instrument determined? 44. State the Atlantic-Pacific Rule for determining significant figures. 45. How many sig ...
... 41. Why is it necessary to perform multiple trials of a scientific experiment? 42. What type of instrument is best for measuring mass, volume, and length? 43. How is the uncertainty of an instrument determined? 44. State the Atlantic-Pacific Rule for determining significant figures. 45. How many sig ...
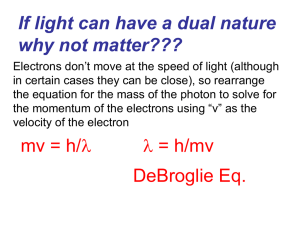
Slide 1
... What would the wavelength of an electron be (9.11 x 10-28 grams) whizzing about it’s orbit in the Bohr model traveling at a speed of 2.0 million meters per second? 3.6 x 10-10 m ...
... What would the wavelength of an electron be (9.11 x 10-28 grams) whizzing about it’s orbit in the Bohr model traveling at a speed of 2.0 million meters per second? 3.6 x 10-10 m ...
Exam 1 Topics to Review (McMurry Chpts 1
... e. Be familiar with general shapes of s, p, and d orbitals. f. Know the number of orbitals in each type of subshell (s, p, d, f subshells), and that a maximum of two electrons can be in each orbital. 7. Electron configurations and orbital diagrams: g. Know how to write both full and condensed ...
... e. Be familiar with general shapes of s, p, and d orbitals. f. Know the number of orbitals in each type of subshell (s, p, d, f subshells), and that a maximum of two electrons can be in each orbital. 7. Electron configurations and orbital diagrams: g. Know how to write both full and condensed ...
10th Grade Chemistry - Ms. Luckasavitch
... 6. State the differences between a scientific theory and a scientific law and provide examples. 7. Discuss the roles of collaboration and communication in the scientific community. 8. Demonstrate use of scientific notation and explain why it is useful. 9. Understand the importance of accuracy and pr ...
... 6. State the differences between a scientific theory and a scientific law and provide examples. 7. Discuss the roles of collaboration and communication in the scientific community. 8. Demonstrate use of scientific notation and explain why it is useful. 9. Understand the importance of accuracy and pr ...
Electron discovered 1897, Thomson Atom model 1913, Bohr
... 1897, Thomson Atom model 1913, Bohr, Rutherford ...
... 1897, Thomson Atom model 1913, Bohr, Rutherford ...
Degeneracy of Hydrogen atom
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.