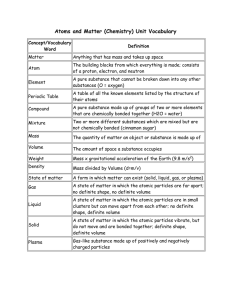
Physical Science Test #1 – Review Guide Properties of Matter
... 19. A __________________________ is made up of more than one kind of atom or molecule in the same place at the same time (but those atoms or molecules are NOT bonded together). 20. ________________________________ are made up of one kind of atom; for example: carbon, oxygen, hydrogen, sodium, or chl ...
... 19. A __________________________ is made up of more than one kind of atom or molecule in the same place at the same time (but those atoms or molecules are NOT bonded together). 20. ________________________________ are made up of one kind of atom; for example: carbon, oxygen, hydrogen, sodium, or chl ...
Slide 1
... People have come to realize that the matter of the world is made from a few fundamental building blocks of nature. The word "fundamental" is key here. By fundamental building blocks we mean objects that are simple and structureless -not made of anything smaller. Even in ancient times, people sought ...
... People have come to realize that the matter of the world is made from a few fundamental building blocks of nature. The word "fundamental" is key here. By fundamental building blocks we mean objects that are simple and structureless -not made of anything smaller. Even in ancient times, people sought ...
Chemistry Curriculum Guide
... Discoveries and insights related to the atom’s structure have changed the model of the atom over time. ...
... Discoveries and insights related to the atom’s structure have changed the model of the atom over time. ...
Notes matter energy
... On the Periodic Table of the Elements (See Week 1 handout), gaseous elements have symbols with an italic font, liquid elements have symbols with an outline font, and solids have symbols with a Times-Roman font. The Law of Definite Composition states that compounds always contain the same proportions ...
... On the Periodic Table of the Elements (See Week 1 handout), gaseous elements have symbols with an italic font, liquid elements have symbols with an outline font, and solids have symbols with a Times-Roman font. The Law of Definite Composition states that compounds always contain the same proportions ...
Static Electricity
... every other electron so they all have the same mass and the same negative charge • The nucleus is composed of positively charged protons and uncharged neutrons • All protons are identical and the charge of the proton is exactly the same size as the charge of the electron, but it is opposite ...
... every other electron so they all have the same mass and the same negative charge • The nucleus is composed of positively charged protons and uncharged neutrons • All protons are identical and the charge of the proton is exactly the same size as the charge of the electron, but it is opposite ...
Announcements
... l Why did electrons have to stay in certain orbitals? l Planets can have any radius in orbiting the Sun It was the wave nature of the electron that determined the l De Broglie suggested nature of the orbits that all particles had a had to be able to fit an integral wave nature as well as a You ...
... l Why did electrons have to stay in certain orbitals? l Planets can have any radius in orbiting the Sun It was the wave nature of the electron that determined the l De Broglie suggested nature of the orbits that all particles had a had to be able to fit an integral wave nature as well as a You ...
Relativity Problem Set 7 - Solutions Prof. J. Gerton October 24, 2011
... In Bohr’s model , the velocity of the electron is quantized as vn = α c/n, where α = 1/137 is the fine structure constant. Since αc = 2.19 × 106 m/s, we see that only the velocities in (b) and (d) are allowed, being given by the above relation with n = 1 and n = 2 respectively. The quantities in (a) ...
... In Bohr’s model , the velocity of the electron is quantized as vn = α c/n, where α = 1/137 is the fine structure constant. Since αc = 2.19 × 106 m/s, we see that only the velocities in (b) and (d) are allowed, being given by the above relation with n = 1 and n = 2 respectively. The quantities in (a) ...
Chemical Equations and Reactions
... with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
... with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
May 2004
... M04E.2—Classical Radiation from a Hydrogen Atom Problem In a naı̈ve classical model of the hydrogen atom’s ground state, the electron moves in a circular orbit of radius r0 = 0.53 × 10−10 m around the center of mass of the electron-proton pair. Since the electron is accelerating, classically it will ...
... M04E.2—Classical Radiation from a Hydrogen Atom Problem In a naı̈ve classical model of the hydrogen atom’s ground state, the electron moves in a circular orbit of radius r0 = 0.53 × 10−10 m around the center of mass of the electron-proton pair. Since the electron is accelerating, classically it will ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
CP Chemistry Final Exam Review Sheet
... temperature change 20. Indicate whether the following can be separated by physical, chemical, or nuclear means. a) atoms ...
... temperature change 20. Indicate whether the following can be separated by physical, chemical, or nuclear means. a) atoms ...
Ch 11 WS Orbitals and Electron Arrangement
... is often thought of as a region of space in which there is a high probability of finding an electron. 8. Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals c. quantum b. quantum mechanical numbers d. principal quantum numbers (n) 9. Principal energ ...
... is often thought of as a region of space in which there is a high probability of finding an electron. 8. Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals c. quantum b. quantum mechanical numbers d. principal quantum numbers (n) 9. Principal energ ...
Reactions I Can..
... 8. Trace the changes in atomic theory starting with Dalton and ending with the modern quantum mechanical model. 9. Describe the basic properties of alpha, beta, and gamma radiation. 10. Explain why some atomic nuclei are unstable 11. Predict the type of nuclear decay that will occur given the compos ...
... 8. Trace the changes in atomic theory starting with Dalton and ending with the modern quantum mechanical model. 9. Describe the basic properties of alpha, beta, and gamma radiation. 10. Explain why some atomic nuclei are unstable 11. Predict the type of nuclear decay that will occur given the compos ...
Atoms
... 8. Trace the changes in atomic theory starting with Dalton and ending with the modern quantum mechanical model. 9. Describe the basic properties of alpha, beta, and gamma radiation. 10. Explain why some atomic nuclei are unstable 11. Predict the type of nuclear decay that will occur given the compos ...
... 8. Trace the changes in atomic theory starting with Dalton and ending with the modern quantum mechanical model. 9. Describe the basic properties of alpha, beta, and gamma radiation. 10. Explain why some atomic nuclei are unstable 11. Predict the type of nuclear decay that will occur given the compos ...
10th Grade Chemistry X (TJ) GRADE(S)/LEVELS SUBJECT Power
... Solutions are mixtures in which particles of one substance are evenly distributed through another substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalin ...
... Solutions are mixtures in which particles of one substance are evenly distributed through another substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalin ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.