Ch 2-1 Properties of Matter
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
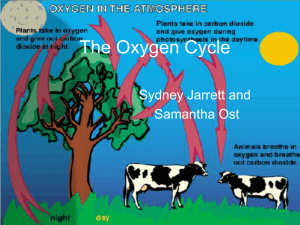
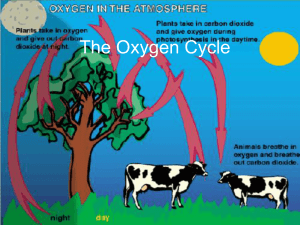
The Oxygen Cycle - EDHSGreenSea.net
... chemical process in which solid rock exposed at earths surface is changed to separate solid particles and dissolved materials ...
... chemical process in which solid rock exposed at earths surface is changed to separate solid particles and dissolved materials ...
REGAN-Emanuel-June2013-FINAL
... Dmc2 = M(232Th) – [ M(228Ra) + M(4He)])c2 Dmc2=0.004382 uc2 = 4.08 MeV 4.08 MeV of ‘binding energy’ from 232Th is released in its decay to 228Ra by the emission of a 4He nucleus (a particle). Due to conservation of linear momentum, this energy is split between the energy of the emitted alpha particl ...
... Dmc2 = M(232Th) – [ M(228Ra) + M(4He)])c2 Dmc2=0.004382 uc2 = 4.08 MeV 4.08 MeV of ‘binding energy’ from 232Th is released in its decay to 228Ra by the emission of a 4He nucleus (a particle). Due to conservation of linear momentum, this energy is split between the energy of the emitted alpha particl ...
Introduction to Chemical Equations
... • You may NOT change any subscripts in any of the reactant’s or product’s formulas ...
... • You may NOT change any subscripts in any of the reactant’s or product’s formulas ...
Chemistry FINAL: CONTENT Review Packet
... a. the total number of protons and neutrons in the nucleus of an atom b. the weighted average mass of the atoms in a naturally occurring sample of an element c. 1/12 the mass of a carbon-12 atom d. the number of protons in the nucleus of an element e. atoms with the same number of protons but differ ...
... a. the total number of protons and neutrons in the nucleus of an atom b. the weighted average mass of the atoms in a naturally occurring sample of an element c. 1/12 the mass of a carbon-12 atom d. the number of protons in the nucleus of an element e. atoms with the same number of protons but differ ...
2A Final Exam Review Worksheet
... o Ideal Gas Law (& d = m/V & M = m/n) o Dalton’s Law (mixture of 2 or more gases) § General Problem § Dalton over water Kinetic Theory of Gases Assumptions & Concepts o 5 assumptions o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average k ...
... o Ideal Gas Law (& d = m/V & M = m/n) o Dalton’s Law (mixture of 2 or more gases) § General Problem § Dalton over water Kinetic Theory of Gases Assumptions & Concepts o 5 assumptions o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average k ...
Quantum Physics - The University of Sydney
... and understand the sections of the textbook specified below, and work through the specified examples. You should attempt as many as possible of the recommended Questions, Exercises and Problems. Problem solving skills can only be acquired by practice. ...
... and understand the sections of the textbook specified below, and work through the specified examples. You should attempt as many as possible of the recommended Questions, Exercises and Problems. Problem solving skills can only be acquired by practice. ...
1 - kurtniedenzu
... 1. The characteristic bright-line spectrum of an element is produced when electrons a. fall back to lower energy levels b. are gained by a neutral atom c. are emitted by the nucleus as beta particles d. move to higher energy levels 2. Compared with an atom of C-12, an atom of C-14 has a. More proton ...
... 1. The characteristic bright-line spectrum of an element is produced when electrons a. fall back to lower energy levels b. are gained by a neutral atom c. are emitted by the nucleus as beta particles d. move to higher energy levels 2. Compared with an atom of C-12, an atom of C-14 has a. More proton ...
1 Chemistry 400: General Chemistry Name: Miller Fall 2015 Final
... involved and their percents of ionization. (8 points) ...
... involved and their percents of ionization. (8 points) ...
Physics Qualifier Part I—Spring 2010 7-Minute Questions α
... b. Linearly polarized light of variable frequency ω is shone on the electron. The potential energy of the electron in the light field is Vf (x,y,t) = Vox cos (ωt). What are the selection rules for transitions between the four lowest energy levels already found? 9. For a particle of mass m in an infi ...
... b. Linearly polarized light of variable frequency ω is shone on the electron. The potential energy of the electron in the light field is Vf (x,y,t) = Vox cos (ωt). What are the selection rules for transitions between the four lowest energy levels already found? 9. For a particle of mass m in an infi ...
FREE Sample Here
... Chemicals used as reagents, such as bromthymol blue or sodium iodide, may permanently stain clothing. Use with caution. ...
... Chemicals used as reagents, such as bromthymol blue or sodium iodide, may permanently stain clothing. Use with caution. ...
chemistry - billpalmer
... 1) Matter is composed of small particles called atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combi ...
... 1) Matter is composed of small particles called atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combi ...
Review Packet
... _____ 58. A number within a formula representing the number of atoms of each element present in the formula. _____ 59. A solid compound dissolved in water. _____ 60. Elements that do not exist alone! Write out a word equation for the following equations. ...
... _____ 58. A number within a formula representing the number of atoms of each element present in the formula. _____ 59. A solid compound dissolved in water. _____ 60. Elements that do not exist alone! Write out a word equation for the following equations. ...
J - X-ray and Observational Astronomy Group
... • Arises due to interaction between nuclear spin angular momentum and the electron total angular momentum. – combined spin angular momentum of nucleus called I • associated nuclear spin quantum number I (0, half-integer or integer) • Iz given by projection quantum number mI which ranges from –I ...
... • Arises due to interaction between nuclear spin angular momentum and the electron total angular momentum. – combined spin angular momentum of nucleus called I • associated nuclear spin quantum number I (0, half-integer or integer) • Iz given by projection quantum number mI which ranges from –I ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.