Quantum Fusion Hypothesis Abstract
... click “HTML”. See the first sentence in the data sheet.) According to Kelly, if there were some way to make 4H at a low enough energy (≤3.53 MeV), then it would undergo β− decay. That can’t be done in an accelerator. When solid-state systems produce 4He, each helium nucleus formed liberates between ...
... click “HTML”. See the first sentence in the data sheet.) According to Kelly, if there were some way to make 4H at a low enough energy (≤3.53 MeV), then it would undergo β− decay. That can’t be done in an accelerator. When solid-state systems produce 4He, each helium nucleus formed liberates between ...
File - Get Involved!
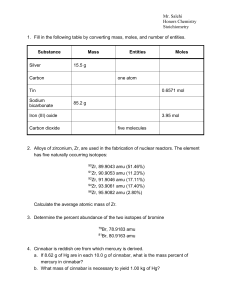
... Atomic Mass Unit (amu) – relative mass of element compared to mass of the C12; each isotope has a different amu Molecular weight (MW) – weighted average of relative abundances of different isotopes of element MW of Element Z = xA(amu A) + xB(amu B) +… ...
... Atomic Mass Unit (amu) – relative mass of element compared to mass of the C12; each isotope has a different amu Molecular weight (MW) – weighted average of relative abundances of different isotopes of element MW of Element Z = xA(amu A) + xB(amu B) +… ...
Chemical Equation
... • Are compounds composed of charged particles. • In general: the electrons are shared between the ions. Metals tend to give up their electrons to an incomplete nonmetal. • All Ionic compounds are represented by their empirical formulas. They are always in the smallest whole number ratios. ...
... • Are compounds composed of charged particles. • In general: the electrons are shared between the ions. Metals tend to give up their electrons to an incomplete nonmetal. • All Ionic compounds are represented by their empirical formulas. They are always in the smallest whole number ratios. ...
Atomic Structure
... Debate in 1600's: Since waves or particles can transfer energy, what is light? Newton – light energy is transferred by particles Huygens – light energy is transferred by waves ...
... Debate in 1600's: Since waves or particles can transfer energy, what is light? Newton – light energy is transferred by particles Huygens – light energy is transferred by waves ...
Atomic Structure
... to study the behavior of small particles going at high speeds. Classical mechanics studies large particles going at relatively slow speeds. Since electrons are small particles going at high speeds the electron (and thus chemistry) can only be understood through the use of quantum mechanics. ...
... to study the behavior of small particles going at high speeds. Classical mechanics studies large particles going at relatively slow speeds. Since electrons are small particles going at high speeds the electron (and thus chemistry) can only be understood through the use of quantum mechanics. ...
Chapter 1 Chemistry: The Study of Matter
... There are more Plasma – high temperature low pressure – electrons separate from nucleus – Most common in the universe More at very low temp – Bose- Einstein condensate – Quantum superfluids ...
... There are more Plasma – high temperature low pressure – electrons separate from nucleus – Most common in the universe More at very low temp – Bose- Einstein condensate – Quantum superfluids ...
PS10_Wk12_122 S17
... (d) Answer the same question (as in (c)) for a nuclide having too many protons. M4. (a) The only stable isotope of fluorine is F-19. Predict possible modes of decay for F-21, F-18, and F-17. (b) Also predict possible modes of decay for 210Po, and (c) 195Au. ...
... (d) Answer the same question (as in (c)) for a nuclide having too many protons. M4. (a) The only stable isotope of fluorine is F-19. Predict possible modes of decay for F-21, F-18, and F-17. (b) Also predict possible modes of decay for 210Po, and (c) 195Au. ...
AP Chemistry Summer Assignment 2016
... b. How many grams of calcium oxide will be produced after 12.25 grams of calcium carbonate are completely decomposed? c. What volume of carbon dioxide gas is produced from 12.25 grams of calcium carbonate at STP? d. What is the volume of carbon dioxide in L if the pressure is 785 mmHg and the temper ...
... b. How many grams of calcium oxide will be produced after 12.25 grams of calcium carbonate are completely decomposed? c. What volume of carbon dioxide gas is produced from 12.25 grams of calcium carbonate at STP? d. What is the volume of carbon dioxide in L if the pressure is 785 mmHg and the temper ...
Dual Nature Of Radiation And Matter
... and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength. ...
... and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength. ...
Atomic Structure - The Student Room
... The number of protons is equal to the atomic number, coincidentally so is the number of electrons in most elements. The number of neutrons can be found by subtracting the proton number from the mass number. ...
... The number of protons is equal to the atomic number, coincidentally so is the number of electrons in most elements. The number of neutrons can be found by subtracting the proton number from the mass number. ...
Nuclear Spin - Home - KSU Faculty Member websites
... spins in ordinary matter. Our bodies have many unpaired protons in H2O. Detect them …... In order to image tissue of various types, Magnetic Resonance Imaging detects the small difference in the numbers of “up” and “down” hydrogen proton spins generated when the object studied is placed in a magneti ...
... spins in ordinary matter. Our bodies have many unpaired protons in H2O. Detect them …... In order to image tissue of various types, Magnetic Resonance Imaging detects the small difference in the numbers of “up” and “down” hydrogen proton spins generated when the object studied is placed in a magneti ...
Chapter 40
... “K” indicate in this notation? a) The characteristic lines involve the n = 1 shell of the molybdenum atoms. b) The K stands for “klystron,” which is the radiation generator. c) The characteristic lines involve the electrons at the highest energy levels within the molybdenum atoms. d) The characteris ...
... “K” indicate in this notation? a) The characteristic lines involve the n = 1 shell of the molybdenum atoms. b) The K stands for “klystron,” which is the radiation generator. c) The characteristic lines involve the electrons at the highest energy levels within the molybdenum atoms. d) The characteris ...
PHYS 305 - Modern Physics (Spring 2016) Department of Physics
... Modern Physics is a undergraduate level course which is intended for students, who have already studied introductory level physics. This course provides a basic introduction to better understanding of special relativity, Quantum mechanics, and applications of quantum theory to: atomic and molecular ...
... Modern Physics is a undergraduate level course which is intended for students, who have already studied introductory level physics. This course provides a basic introduction to better understanding of special relativity, Quantum mechanics, and applications of quantum theory to: atomic and molecular ...
Chapter 13 Notes
... oxidation. Iron is gaining back electrons it had lost to become a free element so it is undergoing reduction. This is called an oxidation-reduction reaction or redox for short. Although this type of reaction is named for oxygen, many other elements undergo redox reactions with each other without oxy ...
... oxidation. Iron is gaining back electrons it had lost to become a free element so it is undergoing reduction. This is called an oxidation-reduction reaction or redox for short. Although this type of reaction is named for oxygen, many other elements undergo redox reactions with each other without oxy ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.