Problem 8: Intermolecular Forces
... Nickel tetracarbonyl, Ni(CO)4, has been used for the purification of Ni metal in the Mond process. Electron counts of these metal carbonyl complexes show that they obey the 18-electron rule. Cobalt and manganese react with CO to form dinuclear complexes Co2(CO)8 and Mn2(CO)10, respectively. (Electro ...
... Nickel tetracarbonyl, Ni(CO)4, has been used for the purification of Ni metal in the Mond process. Electron counts of these metal carbonyl complexes show that they obey the 18-electron rule. Cobalt and manganese react with CO to form dinuclear complexes Co2(CO)8 and Mn2(CO)10, respectively. (Electro ...
Chapter 15 File
... toward the positive electrode. These cathode rays caused the glass to glow when they struck the far side of the tube. The rays could be deflected by a magnetic field. In 1885, after several years of experiments with improved vacuum discharge tubes, William Crookes in England suggested that cathode r ...
... toward the positive electrode. These cathode rays caused the glass to glow when they struck the far side of the tube. The rays could be deflected by a magnetic field. In 1885, after several years of experiments with improved vacuum discharge tubes, William Crookes in England suggested that cathode r ...
chemisty_ass_2
... electron is shielded from the nucleus by the repelling effect of the inner electrons. Across the group, the reverse is the case; the increasing nuclear charge has greater effect. In general, the screening effect by inner electrons is more effective, the closer they are to the nucleus. ii. Distance o ...
... electron is shielded from the nucleus by the repelling effect of the inner electrons. Across the group, the reverse is the case; the increasing nuclear charge has greater effect. In general, the screening effect by inner electrons is more effective, the closer they are to the nucleus. ii. Distance o ...
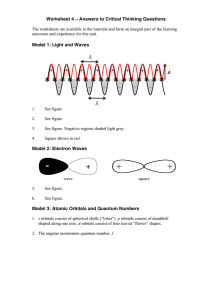
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
Quantum Physics 2 - More About
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
2014-2015 KEY TERMS, DEFINITIONS and FORMULAS for
... number of particles is the same as the number of atoms of carbon in exactly 12 g of carbon-12. Specifically, a mole is 6.022 x 1023 particles of anything. Chp 4-3 97. Orbital – a region in an atom where there is a high probability of finding electrons. 98. Valence electron – an electron that is foun ...
... number of particles is the same as the number of atoms of carbon in exactly 12 g of carbon-12. Specifically, a mole is 6.022 x 1023 particles of anything. Chp 4-3 97. Orbital – a region in an atom where there is a high probability of finding electrons. 98. Valence electron – an electron that is foun ...
Review Questions for 1st year chemistry
... D. The ice becomes tea, physical change Answer: C Ice melting is a physical change from solid to liquid. ...
... D. The ice becomes tea, physical change Answer: C Ice melting is a physical change from solid to liquid. ...
Summer Assignment Ch. 2-5
... Chaperone proteins or chaperonins assist in the proper folding of proteins. Annotate this figure to explain the process. ...
... Chaperone proteins or chaperonins assist in the proper folding of proteins. Annotate this figure to explain the process. ...
Important Equations in Physics (A2) Unit 1: Non-uniform
... high energy electrons, -1 charge, stopped by few mm thick aluminium sheet, weak ionization effect, deflected by electric and magnetic field, proton number increase by one, neutron number decrease by 1 and nucleon number stays the same in parent nuclei electromagnetic radiation, no charge, can only b ...
... high energy electrons, -1 charge, stopped by few mm thick aluminium sheet, weak ionization effect, deflected by electric and magnetic field, proton number increase by one, neutron number decrease by 1 and nucleon number stays the same in parent nuclei electromagnetic radiation, no charge, can only b ...
Study Guide for Final
... Compound – a substance made up of atoms of two or more different elements joined by chemical bonds 1. Cannot be separated by physical means Mixture – a combination of two or more substances that are not chemically combined A. Can be separated by physical means Solution – a single substance composed ...
... Compound – a substance made up of atoms of two or more different elements joined by chemical bonds 1. Cannot be separated by physical means Mixture – a combination of two or more substances that are not chemically combined A. Can be separated by physical means Solution – a single substance composed ...
Vortex-ring-fractal Structure of Hydrogen Atom
... achieved until 1913. In that year the Danish physicist Niels Bohr successfully applied the quantum theory to this problem and created a model of hydrogen. Bohr also discovered a method of calculation of the energy of the stationary states of the hydrogen atom, with use of Planck’s constant h. Later ...
... achieved until 1913. In that year the Danish physicist Niels Bohr successfully applied the quantum theory to this problem and created a model of hydrogen. Bohr also discovered a method of calculation of the energy of the stationary states of the hydrogen atom, with use of Planck’s constant h. Later ...
Electricity
... a great distance from the positive nucleus, giving an overall neutral atom Only certain orbits were allowed (like harmonics in a vibrating violin string) and only a maximum number of electrons could populate each orbit (inner 2, next out 8 etc). These electrons were stable, that is they wouldn’t s ...
... a great distance from the positive nucleus, giving an overall neutral atom Only certain orbits were allowed (like harmonics in a vibrating violin string) and only a maximum number of electrons could populate each orbit (inner 2, next out 8 etc). These electrons were stable, that is they wouldn’t s ...
Objectives: • To see the effect of a magnetic field on a
... Robert Millikan (1868-1953) was able to measure the charge of the electron. Thus, these two experiments determine the mass of the electron. Thomson’s work also formed the basis of the mass spectrometer, which was further developed by Al Neir. Our experiment is a descendent of J. J. Thomson’s origina ...
... Robert Millikan (1868-1953) was able to measure the charge of the electron. Thus, these two experiments determine the mass of the electron. Thomson’s work also formed the basis of the mass spectrometer, which was further developed by Al Neir. Our experiment is a descendent of J. J. Thomson’s origina ...
Barnard Castle School Chemistry Department
... strong and conductors of heat and electricity) or non-metals (which are generally dull, brittle and do not conduct electricity or heat). The particles that make up an element can be either atoms or molecules. A compound is a substance that contains more than one kind of atom chemically joined togeth ...
... strong and conductors of heat and electricity) or non-metals (which are generally dull, brittle and do not conduct electricity or heat). The particles that make up an element can be either atoms or molecules. A compound is a substance that contains more than one kind of atom chemically joined togeth ...
light is a wave
... Electromagnetic waves with different frequencies have different names: radio waves; microwaves; infra red, visible & ultra violet light; X-rays and gamma-rays ...
... Electromagnetic waves with different frequencies have different names: radio waves; microwaves; infra red, visible & ultra violet light; X-rays and gamma-rays ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.