INTRODUCTION TO MINERALOGY 1
... Three ”types” of chemical bonds are the three extreme cases of valenceelectron behaviour in one and the same phenomenon called the chemical bond. ...
... Three ”types” of chemical bonds are the three extreme cases of valenceelectron behaviour in one and the same phenomenon called the chemical bond. ...
Orbitals and energy levels
... The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. This model is based on equations developed by Erwin Schrodinger ...
... The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. This model is based on equations developed by Erwin Schrodinger ...
The Structure of an Atom
... The number of protons in an atom is balanced by the number of electrons in the atom so that the atom does not carry a net charge So, the atomic number also tells us the # of electrons ...
... The number of protons in an atom is balanced by the number of electrons in the atom so that the atom does not carry a net charge So, the atomic number also tells us the # of electrons ...
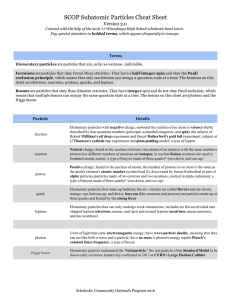
SCOP Subatomic Particles Cheat Sheet
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
Nearly Free Electron Approximation
... Nearly Free Electron Approximation Has had success in accounting for many properties of metals: Electrical conductivity Thermal conductivity Light absorption of metals Wiedemann-Franz law (universal proportionality) However, until quantum theory was incorporated there were problems with predicting s ...
... Nearly Free Electron Approximation Has had success in accounting for many properties of metals: Electrical conductivity Thermal conductivity Light absorption of metals Wiedemann-Franz law (universal proportionality) However, until quantum theory was incorporated there were problems with predicting s ...
atom
... not have to be in the same period) Calculate if it would be easier to gain or lose electrons to get the same number of electrons as the closest ...
... not have to be in the same period) Calculate if it would be easier to gain or lose electrons to get the same number of electrons as the closest ...
Standard 1: Atomic & Molecular Structure
... and neutrons in an atom (units = amu) • Atomic mass (shown on the periodic table): the average mass of all isotopes • Isotope: an atom with the same number of protons and a different number of neutrons • Note: atomic mass generally increases across the periodic table but not always… (look at atomic ...
... and neutrons in an atom (units = amu) • Atomic mass (shown on the periodic table): the average mass of all isotopes • Isotope: an atom with the same number of protons and a different number of neutrons • Note: atomic mass generally increases across the periodic table but not always… (look at atomic ...
Wave Props of Particles - Chemistry at Winthrop University
... from a metal foil of atoms such as gold. (a) If the alpha particles had a kinetic energy of 7.5 MeV, what was their de Broglie wavelength? (b) Explain whether the wave nature of the incident alpha particles should have been taken into account in interpreting these experiments. The mass of an alpha p ...
... from a metal foil of atoms such as gold. (a) If the alpha particles had a kinetic energy of 7.5 MeV, what was their de Broglie wavelength? (b) Explain whether the wave nature of the incident alpha particles should have been taken into account in interpreting these experiments. The mass of an alpha p ...
PHYS150-Ch28
... intrinsic spin. It is useful to compare this to the Earth spinning on its axis. This cannot be truly what is happening since the surface of the electron would be traveling faster than the speed of light. ...
... intrinsic spin. It is useful to compare this to the Earth spinning on its axis. This cannot be truly what is happening since the surface of the electron would be traveling faster than the speed of light. ...
Atomic Structure | Topic Notes
... Thomson’s Charge, e/m Ratio • showed cathode rays were attracted to positive plate - ∴ negative • measured e/m ratio using the fact that they are deflected by magnetic fields • same e/m no matter what gas/electrode materials - ∴ in all matter ...
... Thomson’s Charge, e/m Ratio • showed cathode rays were attracted to positive plate - ∴ negative • measured e/m ratio using the fact that they are deflected by magnetic fields • same e/m no matter what gas/electrode materials - ∴ in all matter ...
Electrons in a Shell - University of California, Berkeley
... This pressure acts to compress the layer. The compression, however, is limited by the Fermi pressure - the consequence of the Pauli exclusion principle forbidding more than one electron in a given quantum state. The number of available electron states per unit volume (which is equal to the electron ...
... This pressure acts to compress the layer. The compression, however, is limited by the Fermi pressure - the consequence of the Pauli exclusion principle forbidding more than one electron in a given quantum state. The number of available electron states per unit volume (which is equal to the electron ...
File
... In Schrodinger’s model, there are four “quantum” numbers that tell us where an electron is likely to be located. Principal (n), 1-7, gives the energy level Subshell (l), s-p-d-f, gives the shape of region Orbital (m), gives the orientation in space of the shapes Spin (s), clockwise or coun ...
... In Schrodinger’s model, there are four “quantum” numbers that tell us where an electron is likely to be located. Principal (n), 1-7, gives the energy level Subshell (l), s-p-d-f, gives the shape of region Orbital (m), gives the orientation in space of the shapes Spin (s), clockwise or coun ...
About Electricity - CBE Project Server
... circuit. In order to wor:<, a circuit needs a source of electrical energy. Batteries are often used to supply electrical energy. In a battery, electrical energy flows from the negative terminal through the circuit to the positive terminal. A battery is made up of one or more cells that are connected ...
... circuit. In order to wor:<, a circuit needs a source of electrical energy. Batteries are often used to supply electrical energy. In a battery, electrical energy flows from the negative terminal through the circuit to the positive terminal. A battery is made up of one or more cells that are connected ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.