Monday, March 2, 2015
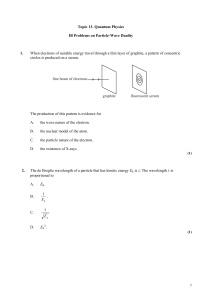
... These electrons are focused by the cathode structure into a beam and are accelerated by potential differences of thousands of volts until they impinge on a metal anode surface, producing x rays by bremsstrahlung as they stop in the anode material X-ray wavelengths range 0.01 – 10nm. What is the mini ...
... These electrons are focused by the cathode structure into a beam and are accelerated by potential differences of thousands of volts until they impinge on a metal anode surface, producing x rays by bremsstrahlung as they stop in the anode material X-ray wavelengths range 0.01 – 10nm. What is the mini ...
Chapter 5
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
ppt - LPSC
... We find out that this low efficiency is due to the cell isolation cut in ATLFast but the paper is not so clear : “ In order to define isolated electrons, the following criteria are applied : the difference in the energy in a cone of DR=0.2 around the electron direction and the smeared electron energ ...
... We find out that this low efficiency is due to the cell isolation cut in ATLFast but the paper is not so clear : “ In order to define isolated electrons, the following criteria are applied : the difference in the energy in a cone of DR=0.2 around the electron direction and the smeared electron energ ...
Set #4 - comsics
... mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separation, r, in the ground state orbit of positronium? ...
... mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separation, r, in the ground state orbit of positronium? ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
... When an electron in an excited state returns to a lower energy state, it emits a photon of energy, which may be observed as light. ...
Atomic Structure Note Page
... a. The overall (net) charge of the nucleus is positive. i. The overall (net) charge of the nucleus is equal to the number of protons. b. There is an attractive force between protons and electrons. i. Opposites attract and like charges repel one another. c. Atoms have a Neutral Charge because the num ...
... a. The overall (net) charge of the nucleus is positive. i. The overall (net) charge of the nucleus is equal to the number of protons. b. There is an attractive force between protons and electrons. i. Opposites attract and like charges repel one another. c. Atoms have a Neutral Charge because the num ...
Light and Electrons!
... -Some claimed that light had to be particles because there was evidence of it going around objects; also Photoelectric Effect by Einstein helped the cause -Photoelectric Effect theorized that light has photons, or “packets” of energy -A man named Thomas Young proved, however, that light acts in wave ...
... -Some claimed that light had to be particles because there was evidence of it going around objects; also Photoelectric Effect by Einstein helped the cause -Photoelectric Effect theorized that light has photons, or “packets” of energy -A man named Thomas Young proved, however, that light acts in wave ...
Unit 5 File
... Atom- the basic particle from which all elements are made Theory-an unifying explanation for a broad range of hypotheses and observations Electron- a tiny, negatively charged particle that moves around the outside of the nucleus of an atom Model-a representation of a complex object or process, used ...
... Atom- the basic particle from which all elements are made Theory-an unifying explanation for a broad range of hypotheses and observations Electron- a tiny, negatively charged particle that moves around the outside of the nucleus of an atom Model-a representation of a complex object or process, used ...
Chapter 8: Periodic Properties of the Elements
... electrons means electrons in the 2s sublevel experience a greater attractive force to the nucleus and are not shielded as effectively • Penetration causes the energies of sublevels in the same principal level to not be degenerate (2s and 2p are different energies) • In the 4th and 5th principle leve ...
... electrons means electrons in the 2s sublevel experience a greater attractive force to the nucleus and are not shielded as effectively • Penetration causes the energies of sublevels in the same principal level to not be degenerate (2s and 2p are different energies) • In the 4th and 5th principle leve ...
Characteristics of Waves
... There are ____________, named after the scientists that discovered them, that govern the filling of these orbitals with electrons… ...
... There are ____________, named after the scientists that discovered them, that govern the filling of these orbitals with electrons… ...
QUANTUM MECHANICAL MODEL OF THE ATOM
... the study of the motions of the microscopic objects that have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does n ...
... the study of the motions of the microscopic objects that have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does n ...
Wave-particle_duality
... (ii) State and explain what would happen to this pressure if the light is reflected rather than absorbed by the surface. ...
... (ii) State and explain what would happen to this pressure if the light is reflected rather than absorbed by the surface. ...
PS#4
... may have values between the sum of the l values of the individual electrons and the absolute value of the difference of these numbers. L l1 l 2 , l1 l 2 1,..., l1 l 2 6. The quantum number S represents the total spin angular momentum, and describes the s-s coupling of the spin angular momen ...
... may have values between the sum of the l values of the individual electrons and the absolute value of the difference of these numbers. L l1 l 2 , l1 l 2 1,..., l1 l 2 6. The quantum number S represents the total spin angular momentum, and describes the s-s coupling of the spin angular momen ...
Charge to mass ratio of electron
... which is attached to the back of the rear Helmholtz coil. By lining up the electron beam with its mirror image in the mirrored scale, you can measure the radius of the beam path without parallax error. Since the beam has a finite width, the measurement should be repeated a sufficient number of times ...
... which is attached to the back of the rear Helmholtz coil. By lining up the electron beam with its mirror image in the mirrored scale, you can measure the radius of the beam path without parallax error. Since the beam has a finite width, the measurement should be repeated a sufficient number of times ...
Models of an atom and old quantum theory
... are close-packed in a solid foil, so that the individual atom's clouds of positive charge would more or less touch, leading to a roughly uniform positive charge distribution inside the material. Electric elds produced by such a distribution of charge are too small to de ect a fast and heavy α parti ...
... are close-packed in a solid foil, so that the individual atom's clouds of positive charge would more or less touch, leading to a roughly uniform positive charge distribution inside the material. Electric elds produced by such a distribution of charge are too small to de ect a fast and heavy α parti ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.