John Dalton Atomic Model
... • Rutherford shot alpha particles at a piece of gold foil surrounded by detectors • This suggests that they hit something larger = Nucleus • Gold Foil Video Demonstration ...
... • Rutherford shot alpha particles at a piece of gold foil surrounded by detectors • This suggests that they hit something larger = Nucleus • Gold Foil Video Demonstration ...
ENT145/3 Materials Engineering Tutorial 1 (Answer) 1. Why is it
... Atomic structure relates to the number of protons and neutrons in the nucleus of an atom, as well as the number and probability distributions of the constituent electrons. Crystal structure pertains to the arrangement of atoms in the crystalline solid material. ...
... Atomic structure relates to the number of protons and neutrons in the nucleus of an atom, as well as the number and probability distributions of the constituent electrons. Crystal structure pertains to the arrangement of atoms in the crystalline solid material. ...
All transitions ending in the ground state, produce photons in what
... will be uncertain. In a high pressure gas, frequent collisions stimulate atoms in excited states to de-excite and emit photons almost immediately. This shortening of the lifetime produces an small variation in the emitted photon’s energy. As a consequence, the width of spectral lines can directly re ...
... will be uncertain. In a high pressure gas, frequent collisions stimulate atoms in excited states to de-excite and emit photons almost immediately. This shortening of the lifetime produces an small variation in the emitted photon’s energy. As a consequence, the width of spectral lines can directly re ...
Chapter 31 Fall 2010 v1
... In 1879 Edwin Hall (a graduate student at Johns Hopkins U.) devised an experiment that distinguishes between positive and negative charge carriers in a conductor. A magnetic field perpendicular to an electric current produces a electric potential difference that depends on the sign of the charge car ...
... In 1879 Edwin Hall (a graduate student at Johns Hopkins U.) devised an experiment that distinguishes between positive and negative charge carriers in a conductor. A magnetic field perpendicular to an electric current produces a electric potential difference that depends on the sign of the charge car ...
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... gold foils. Alpha particles are helium atoms minus their electrons, so they have a charge of +2e. In the Thomson model, the electric charge is smeared out over the atomic volume, and no (or very weak) interaction is expected between the charged alpha particles and the gold atoms. That's because ther ...
... gold foils. Alpha particles are helium atoms minus their electrons, so they have a charge of +2e. In the Thomson model, the electric charge is smeared out over the atomic volume, and no (or very weak) interaction is expected between the charged alpha particles and the gold atoms. That's because ther ...
Electron Configurations
... 1) Rutherford’s model is great for showing where the protons and neutrons in an atom are, but it did not given any information about where to find the electrons or why the negative electrons did not just get stuck to the positive nucleus. ...
... 1) Rutherford’s model is great for showing where the protons and neutrons in an atom are, but it did not given any information about where to find the electrons or why the negative electrons did not just get stuck to the positive nucleus. ...
Electron Diffraction
... Use the graphical method to find the average values for the distances between the atomic planes in the graphite crystal d11 (outer ring) and d10 (inner ring). To determine the combination of the experimental parameters that is proportional to d, one need to substitute the expression for the electron ...
... Use the graphical method to find the average values for the distances between the atomic planes in the graphite crystal d11 (outer ring) and d10 (inner ring). To determine the combination of the experimental parameters that is proportional to d, one need to substitute the expression for the electron ...
The Quantum Mechanical Model of the Atom
... the attraction between the proton and electron and the kinetic energy of the moving electron) • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a particular value of E. – A specific wave function is often called an orbital ...
... the attraction between the proton and electron and the kinetic energy of the moving electron) • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a particular value of E. – A specific wave function is often called an orbital ...
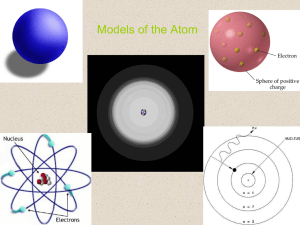
Models of the Atom
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
CHAPTER 3: The Experimental Basis of Quantum
... Planck assumed that the radiation in the cavity was emitted (and absorbed) by some sort of “oscillators.” He used Boltzman’s statistical methods to arrive at the following formula that fit the blackbody radiation data. ...
... Planck assumed that the radiation in the cavity was emitted (and absorbed) by some sort of “oscillators.” He used Boltzman’s statistical methods to arrive at the following formula that fit the blackbody radiation data. ...
Worksheets for Chapter 7
... 5. Write the electron configuration code for phosphorus. 6. How many valence electrons does phosphorus have? 7. Write the electron configuration code for cobalt. 8. How many valence electrons does cobalt have? 9. Write the electron configuration code for bromine. 10. How many valence electrons does ...
... 5. Write the electron configuration code for phosphorus. 6. How many valence electrons does phosphorus have? 7. Write the electron configuration code for cobalt. 8. How many valence electrons does cobalt have? 9. Write the electron configuration code for bromine. 10. How many valence electrons does ...
(n=1).
... En= -13.6 Z2/n2 • Photon emitted when electron jumps from high energy to low energy orbit. Photon absorbed when electron jumps from low energy to high energy: | E1 – E2 | = h f = h c / l ...
... En= -13.6 Z2/n2 • Photon emitted when electron jumps from high energy to low energy orbit. Photon absorbed when electron jumps from low energy to high energy: | E1 – E2 | = h f = h c / l ...
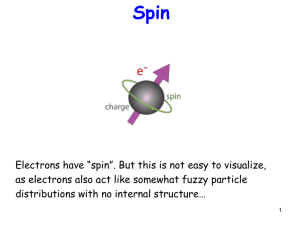
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.