The Quantum Mechanical Model and Electron
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
Lecture IV : Feb 8, 2016 Learning from Two Hole Experiment (A
... Young’s double slit interferometer ( two-hole setup) introduced about two hundred years ago, is one of the most versatile tool to distinguish waves and particle Diffractions through crystals, where atoms are arranged in a periodic pattern provide a multi-hole analog of the double-hole set up. The de ...
... Young’s double slit interferometer ( two-hole setup) introduced about two hundred years ago, is one of the most versatile tool to distinguish waves and particle Diffractions through crystals, where atoms are arranged in a periodic pattern provide a multi-hole analog of the double-hole set up. The de ...
Print version
... The electron at the top most level has a non zero energy at T = 0 called Ef (Fermi energy) When you apply an Electric field the net effect is shifting electrons near the fermi level The electron is effectively a wave and the mean free path is dependent on deviations from a perfect lattice not classi ...
... The electron at the top most level has a non zero energy at T = 0 called Ef (Fermi energy) When you apply an Electric field the net effect is shifting electrons near the fermi level The electron is effectively a wave and the mean free path is dependent on deviations from a perfect lattice not classi ...
PHY583 - Note 1e - Free Electron Theory of Metal
... This discrepancy implies that we are using the wrong value for L & that the scattering sites for electrons are not adjacent ion cores but more widely separated scattering centres. We can account for the unexpectedly long electron mean free path by taking the wave nature of the electron into account. ...
... This discrepancy implies that we are using the wrong value for L & that the scattering sites for electrons are not adjacent ion cores but more widely separated scattering centres. We can account for the unexpectedly long electron mean free path by taking the wave nature of the electron into account. ...
Slides from lecture 4.
... the very fast. For everyday objects much larger and much more massive than atoms and much slower than the speed of light, classical physics does a great job. ...
... the very fast. For everyday objects much larger and much more massive than atoms and much slower than the speed of light, classical physics does a great job. ...
Lesson 3.2 Defining the Atom
... magnetic and electric fields on the cathode ray to determine the charge-to-mass ratio of a charged particle, then compared it to known values. • The mass of the charged particle was much less than a hydrogen atom, then the lightest known atom. • Thomson received the Nobel Prize in 1906 for identifyi ...
... magnetic and electric fields on the cathode ray to determine the charge-to-mass ratio of a charged particle, then compared it to known values. • The mass of the charged particle was much less than a hydrogen atom, then the lightest known atom. • Thomson received the Nobel Prize in 1906 for identifyi ...
quantum, relativistic and classical physics
... momentum S which occurs when an atom is placed in a magnetic field aligned along the z direction. In this connection explain the role of the quantum numbers m and ms and define how L and S are related to the corresponding quantum numbers and s. [7 marks] (ii) Briefly note the experimental observa ...
... momentum S which occurs when an atom is placed in a magnetic field aligned along the z direction. In this connection explain the role of the quantum numbers m and ms and define how L and S are related to the corresponding quantum numbers and s. [7 marks] (ii) Briefly note the experimental observa ...
Chapter Summary
... referred to as “spin up” and “spin down.” The spin angular momentum is characterized by the spin quantum number, which can take on values of +1/2 or –1/2. Understanding the periodic table of elements One key to understanding the periodic table is the Pauli exclusion principle – no two electrons in a ...
... referred to as “spin up” and “spin down.” The spin angular momentum is characterized by the spin quantum number, which can take on values of +1/2 or –1/2. Understanding the periodic table of elements One key to understanding the periodic table is the Pauli exclusion principle – no two electrons in a ...
ch04_sec3_as - LCMR School District
... electrons are located. Each energy level may contain only a certain number of electrons. The electrons in an atom’s outer energy level are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four e ...
... electrons are located. Each energy level may contain only a certain number of electrons. The electrons in an atom’s outer energy level are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four e ...
E04 Atomic, Nuclear, and Particle Physics Chapter 7. Atomic
... The nucleus can lose energy by emitting radiation. There are three types of ionizing radiation: α, β and g. Alpha and beta emissions result in a change in the number of protons and neutrons. Gamma is a form of electromagnetic radiation, similar to X-rays. When a nucleus changes in this way it is sai ...
... The nucleus can lose energy by emitting radiation. There are three types of ionizing radiation: α, β and g. Alpha and beta emissions result in a change in the number of protons and neutrons. Gamma is a form of electromagnetic radiation, similar to X-rays. When a nucleus changes in this way it is sai ...
Energy Levels and Light Absorption
... angular momentum – Particles act like spinning tops – Spin is quantized ( like energy, angular momentum comes in discrete units) in units of – Particles like electrons have ½ integer spin – fermions – Particles like photons have integer spin – bosons ...
... angular momentum – Particles act like spinning tops – Spin is quantized ( like energy, angular momentum comes in discrete units) in units of – Particles like electrons have ½ integer spin – fermions – Particles like photons have integer spin – bosons ...
atoms. molecules, and ions
... discovered by Henri Bequerel. In 1898, Rutherford discovered 3 different types of radioactivity: a, b, g He later used the alpha particles in a famous experiment that ended with the discovery of the “nuclear ...
... discovered by Henri Bequerel. In 1898, Rutherford discovered 3 different types of radioactivity: a, b, g He later used the alpha particles in a famous experiment that ended with the discovery of the “nuclear ...
Name
... The Quantum Mechanical Model The quantum mechanical model determines how likely it is to find an electron in various locations around the atom. The quantum mechanical model is based on mathematics, not on experimental evidence. This model does not specify an exact path an electron takes around the n ...
... The Quantum Mechanical Model The quantum mechanical model determines how likely it is to find an electron in various locations around the atom. The quantum mechanical model is based on mathematics, not on experimental evidence. This model does not specify an exact path an electron takes around the n ...
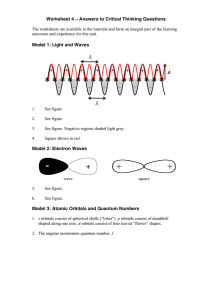
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
Chapter 6 Quiz
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.