Physics 141 Mechanics Yongli Gao Lecture 4 Motion in 3-D
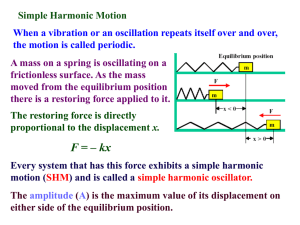
... • You may not know it, but every atom/molecule in your body is oscillating. • For any system, there's at least one state that the system is of the lowest potential energy. This is a point of stable equilibrium, or the bottom of the valley in a potential vs. position curve. • If the system is of a sm ...
... • You may not know it, but every atom/molecule in your body is oscillating. • For any system, there's at least one state that the system is of the lowest potential energy. This is a point of stable equilibrium, or the bottom of the valley in a potential vs. position curve. • If the system is of a sm ...
Light
... If light moves, how fast is it? Galileo tried to measure the speed of light using signals from lanterns on two mountains. He was unsuccessful – light is too fast! The first measurement of the speed of light was astronomical, made by Roemer in 1676 using the eclipses of the moons of Jupiter (which h ...
... If light moves, how fast is it? Galileo tried to measure the speed of light using signals from lanterns on two mountains. He was unsuccessful – light is too fast! The first measurement of the speed of light was astronomical, made by Roemer in 1676 using the eclipses of the moons of Jupiter (which h ...
MS PowerPoint - Catalysis Eprints database
... The effect of absorption of radiation on the transparent material is to change is from a low energy state (called the ground state) to a higher energy state (called the excited state). The difference between all the spectroscopic techniques is that they use different wavelength radiation that has di ...
... The effect of absorption of radiation on the transparent material is to change is from a low energy state (called the ground state) to a higher energy state (called the excited state). The difference between all the spectroscopic techniques is that they use different wavelength radiation that has di ...
Non-stationary States and Electric Dipole Transitions Evolution in
... In this section we will try to understand, at least qualitatively, how a molecule interacts with light (electromagnetic radiation). You will recall that electromagnetic radiation is associated with electric and magnetic fields which vary with time. If these fields are not very strong, we may treat t ...
... In this section we will try to understand, at least qualitatively, how a molecule interacts with light (electromagnetic radiation). You will recall that electromagnetic radiation is associated with electric and magnetic fields which vary with time. If these fields are not very strong, we may treat t ...
18.1 Electromagnetic Waves
... Red light or infrared rays, no matter how bright, does not cause electrons to be emitted from this metal surface. When blue light or ultraviolet rays strike the metal surface, electrons are emitted, even if the light is dim. ...
... Red light or infrared rays, no matter how bright, does not cause electrons to be emitted from this metal surface. When blue light or ultraviolet rays strike the metal surface, electrons are emitted, even if the light is dim. ...
Energy
... charges. Can be either potential or kinetic depending on if they are in motion or being stored. • Examples: 1)Lightning 2)Getting shocked by doorknob 3)Power outlet ...
... charges. Can be either potential or kinetic depending on if they are in motion or being stored. • Examples: 1)Lightning 2)Getting shocked by doorknob 3)Power outlet ...
The Atom
... Dalton and Thompson started serious research on the atom. Dalton suggested that elements combine in certain proportions because they are made of single atoms. Thompson discovered that there are small particles inside the atom. Thompson thought that electrons were mixed throughout an atom, like the p ...
... Dalton and Thompson started serious research on the atom. Dalton suggested that elements combine in certain proportions because they are made of single atoms. Thompson discovered that there are small particles inside the atom. Thompson thought that electrons were mixed throughout an atom, like the p ...
Chapter 2
... one molecule and an electronegative atom of another molecule Common between dipoles such as water Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...
... one molecule and an electronegative atom of another molecule Common between dipoles such as water Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape ...