Homework for the electron microscopy class
... The wavelength of photons is given by the expression =hc/E = 12396 eV- /E where h is Planck’s constant and c is the speed of light (in the medium). For electrons, the equivalent expression is = h/p where p is the electron momentum: p = mv. In classical mechanics the energy of a particle is given ...
... The wavelength of photons is given by the expression =hc/E = 12396 eV- /E where h is Planck’s constant and c is the speed of light (in the medium). For electrons, the equivalent expression is = h/p where p is the electron momentum: p = mv. In classical mechanics the energy of a particle is given ...
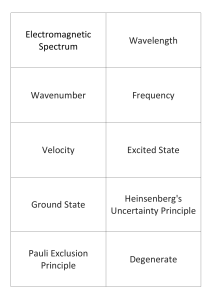
Unit 1 Inorganic Flashcards
... substance. It can also be described as an increase in oxidation number. ...
... substance. It can also be described as an increase in oxidation number. ...
Electromagnetic Spectrum Wavelength Wavenumber Frequency
... substance. It can also be described as an increase in oxidation number. ...
... substance. It can also be described as an increase in oxidation number. ...
THINGSYOUNEEDTOKNOW-modern
... ELECTRONS can collide with PHOTONS. Their combined momentums and energies are conserved. If Electron losses energy, thus losses speed If photon losses energy, its frequency decreases Because photon is light and must travel at c. The sum of all mass + energy is conserved in the universe. The sum of a ...
... ELECTRONS can collide with PHOTONS. Their combined momentums and energies are conserved. If Electron losses energy, thus losses speed If photon losses energy, its frequency decreases Because photon is light and must travel at c. The sum of all mass + energy is conserved in the universe. The sum of a ...
Student Presentation
... integers called “quantum numbers”. – Energy is “quantized” meaning it can only exist at certain energy levels and not in between • Like rungs on a ladder • Correspondence Principle ...
... integers called “quantum numbers”. – Energy is “quantized” meaning it can only exist at certain energy levels and not in between • Like rungs on a ladder • Correspondence Principle ...
Topic 1 - The Nature of Light
... where h is a constant (Planck’s constant, h ≈ 6.63 x 10-34 Js) f, λ, c, are frequency, wavelength and velocity of light (in vacuum) respectively. ...
... where h is a constant (Planck’s constant, h ≈ 6.63 x 10-34 Js) f, λ, c, are frequency, wavelength and velocity of light (in vacuum) respectively. ...
Physics 280/Jones Week 02 In-Class Problems Fall 2014 1
... 5. An interesting proposition. Imagine reversing the photoelectric effect. Imagine that the voltage between the plates is off when light strikes one plate and liberates an electron, but before the electron reaches the second plate, the voltage is increased to stopping potential so that the electron ...
... 5. An interesting proposition. Imagine reversing the photoelectric effect. Imagine that the voltage between the plates is off when light strikes one plate and liberates an electron, but before the electron reaches the second plate, the voltage is increased to stopping potential so that the electron ...
Wave – Particle Duality of Energy and Matter
... The remarkable aspects of the photoelectric effect when it was first observed were: 1. The electrons were emitted immediately - no time lag! 2. Increasing the intensity of the light increased the number of photoelectrons, but not their maximum kinetic energy! 3. Red light will not cause the ejection ...
... The remarkable aspects of the photoelectric effect when it was first observed were: 1. The electrons were emitted immediately - no time lag! 2. Increasing the intensity of the light increased the number of photoelectrons, but not their maximum kinetic energy! 3. Red light will not cause the ejection ...
Chapter 38
... (c) What was the initial speed of the alpha particle? KE = ½ mv2 = 5.82x10-13 J for the alpha particle m = 6.64×10−27 kg, so v= 1.32 x 107 m/s = 0.044c γ = [1 – (v/c)2]-1/2 = 1.00097 non-relativistic is ok ...
... (c) What was the initial speed of the alpha particle? KE = ½ mv2 = 5.82x10-13 J for the alpha particle m = 6.64×10−27 kg, so v= 1.32 x 107 m/s = 0.044c γ = [1 – (v/c)2]-1/2 = 1.00097 non-relativistic is ok ...
Mass of electron m = 9.1. 10 kg
... Formulae and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...
... Formulae and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...